Characteristics and Outcomes of COVID-19 Patients Admitted to Livingstone Teaching Hospital, Zambia: A Cross-Sectional Analysis (January 2020- September 2021)
Precious Simushi
Zambia National Public Health Institute
Mowa Zambwe
2Benefits Department, Workers Compensation Fund Control Board, Lusaka, Zambia
Peter Jay Chipimo
World Health Organization
DOI: https://doi.org/10.55320/mjz.52.5.815
Keywords:COVID-19, hypertension, diabetes mellitus
ABSTRACT
Background: COVID-19, an acute respiratory infectious disease transmitted through the respiratory tract, is a strain of coronaviruses and of zoonotic origins. The virus is widespread and has been declared a globe pandemic. As a result of no prior immunological exposure to SARS-COV-2, the human population is vulnerable to infection and disease. It is as such imperative that empirical evidence be provided bases on data from admitting Health facilities.
Objective: To determine the characteristics and outcomes of COVID-19 patients admitted at Livingstone Teaching Hospital.
Methods: This was a cross-sectional study that involved reviewing the hospital records of COVID-19 patients at Livingstone Teaching Hospital in Zambia's Southern Province. A structured data collection form was used to gather information on patient demographics, clinical characteristics, and comorbidities.
Results: From a total of 355 clients, 222 (62.54%) were male and 133 (37.46%) were female. Discharged 274 (77.18%), while 81 (22.82%) died. Among those who died were the older clients with a median age of 65 (p <0.001). The median age was 48.5 years. Patients presented with a cough 180 (50.7%), chest pain 123 (34.65%) and shortness of breath 121 (34.04%). Statistically significant comorbidities recorded included Hypertension 121 (34.08%), Diabetes mellitus 69 (19.44%), and HIV 38 (10.7%). The most prevalent underlying condition observed was hypertension 121 (34.08).
Conclusion: Older age was associated with greater odds of COVID-19 infection. The main clinical manifestations of COVID-19 were cough, chest pain and shortness of breath. Our study findings highlight that older age, HIV positive status and the presence of Diabetes Mellitus are independent risk factors even after adjusting for other variables.
INTRODUCTION
The Severe acute respiratory syndrome coronavirus 2 (Sars-CoV-2) responsible for causing the COVID-19 infection is a novel Coronavirus. It is of zoonotic origins and belongs to the beta coronavirus genera with similar phylogenetic characteristics as the SARS-CoV.[1] It was described in December, 2019 by the World Health Organisation (WHO) as an acute respiratory disease infection responsible for a cluster of atypical pneumonia cases reported two weeks earlier in Wuhan city, Hubei province, in China.[1] [2] [3]
WHO indicated the COVID-19 pandemic to be of public health emergency and of international concern that needed extensive resources directed at containing and controlling the spread of the virus as a surge in the number of cases was observed.[1] [2] The severity of some of the cases of Covid-19 infection mimicked that of SARS-CoV, however, it was observed that the SARS-CoV-2 virus is not similar to other coronaviruses that usually spread in human beings responsible for causing common cold to severe respiratory distress syndrome.[4] [5] 4,5 The SARS-CoV-2 has shown to surpass SARS and MERS in terms of the number of people infected and the spatial range of epidemic areas.
By the second week of June the number of COVID-19 cases surpassed 200,000 and had escalated to 400,000 by the 6th of July, 2020.6 On the 14th of February, 2020 and 16th of March, 2020 the first case of Sars-cov-2 infection was reported in Egypt and Zambia respectively.[7] [8] 7,8 As opposed to the prediction by public health experts on COVID-19 in Africa, worst outcomes, the incidences, hospitalization and mortality rates recorded have been to a minimal as compared to other continents.[6]
Human population at the time of the pandemic had no direct immunological experience with Sars-CoV-2, leaving the population vulnerable to infection and disease hence the devastating outcomes experienced globally.[9] [10] [11] This led to a surge in cases recorded, hospitalization and an elevation in mortality in the country. The objective of this study was therefore to determine the characteristics of Covid-19 patients at Livingstone Teaching Hospital.
METHODS
Study design
This was a cross-sectional analysis of secondary hospital data. The data was collected using a structured data collection form. Medical records and data for the admitted clients with laboratory confirmed Covid-19 PCR and/or RDT positive results recorded from January 2020 to September 2021 were used.
Sample size
A limitation of this retrospective, cross-sectional study is the absence of a prior sample size calculation based on power analysis. This decision was pragmatic, allowing us to include all available COVID-19 patient admissions with complete data at Livingstone Teaching Hospital from January 1, 2020, to September 30, 2021. This approach aimed to comprehensively capture the characteristics and outcomes of all accessible patients during the defined study period.
Sampling technique
All COVID-19 patients’ records admitted to Livingstone Teaching Hospital between January 1, 2020, and September 30, 2021, were screened for inclusion. Records were considered eligible if they had a confirmed COVID-19 diagnosis (via PCR or RDT) and complete demographic and clinical data available in their hospital records. Patients who were not admitted or those with missing PCR/RDT results were excluded from the study.
Data collection
A structured data collection form was utilized to abstract data from eligible patient hospital records. The form was designed to capture demographic information such as age, sex, clinical characteristics including presenting symptoms, admission date, outcome, and pre-existing comorbidities as documented within the patient's medical file. These comorbidities included, but were not limited to, HIV, hypertension, diabetes mellitus, and chronic respiratory diseases. Data extractors were trained on the use of the form to ensure consistency.
To enhance the reliability of data and minimize potential for information bias during the abstraction process, several steps were undertaken. All data extractors received standardized training on the use of the data collection form and definitions of all variables. A detailed data extraction protocol was established, guiding the consistent interpretation and recording of information from patient records. Additionally, periodic checks were performed by a senior researcher on a subset of extracted data to ensure accuracy and consistency across extractors.
Statistical analysis
Data was analysed using STATA 64 to produce descriptive statistics. Mann-Whitney and t-test were used on continuous independent variables between those with and those without outcome. Chi-square test was used to determine associations between two categorical variables. Logistic regression was used to control for confounding and determine the contribution of each variable towards the outcome. Sub analysis of the outcome variable was conducted to determine the relationship between each underlying condition and the predicting variables.
RESULTS
Basic characteristics of study population
Table I, indicates 222 (62.54%) were male and 133 (37.46%) were female and a median age was 48.5 was recorded. Upon admission clinical presentation were recorded for all clients that were confirmed positive with the Sars-Cov-2. Significant characteristic observed on admission included cough 180 (50.7%), followed by fever 138 (38.87%) then chest pain with 123 (34.65%). From the study population of 355 clients, significant comorbidities; HIV positive clients recorded were 38 (10.7%), hypertension 121 (34.08), Diabetes mellitus clients were 69 (19.44%). Most of the cases were hypertensive, followed by the diabetics and the HIV positives.
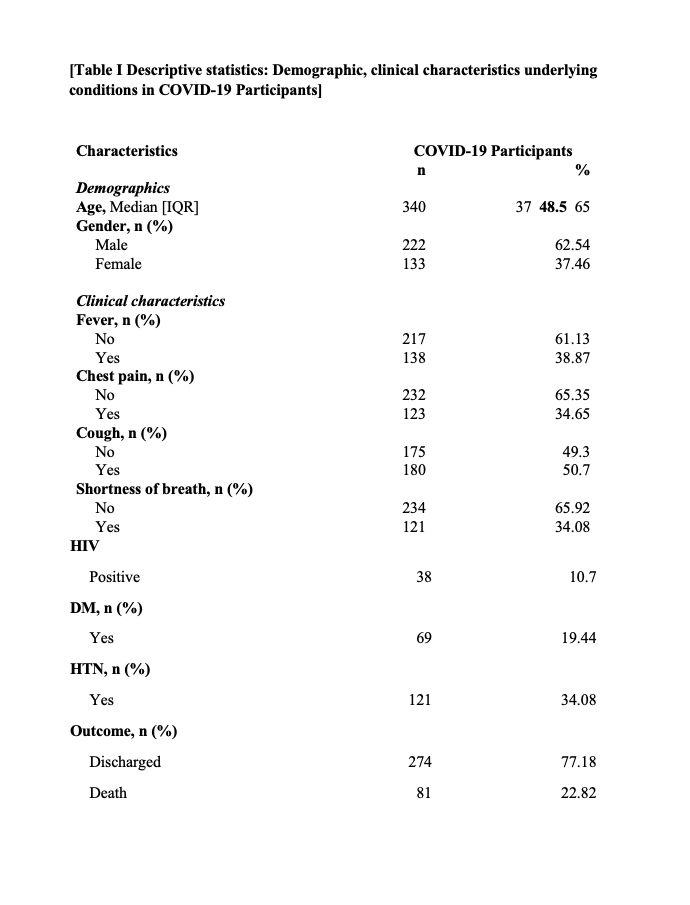
Demographics and outcomes
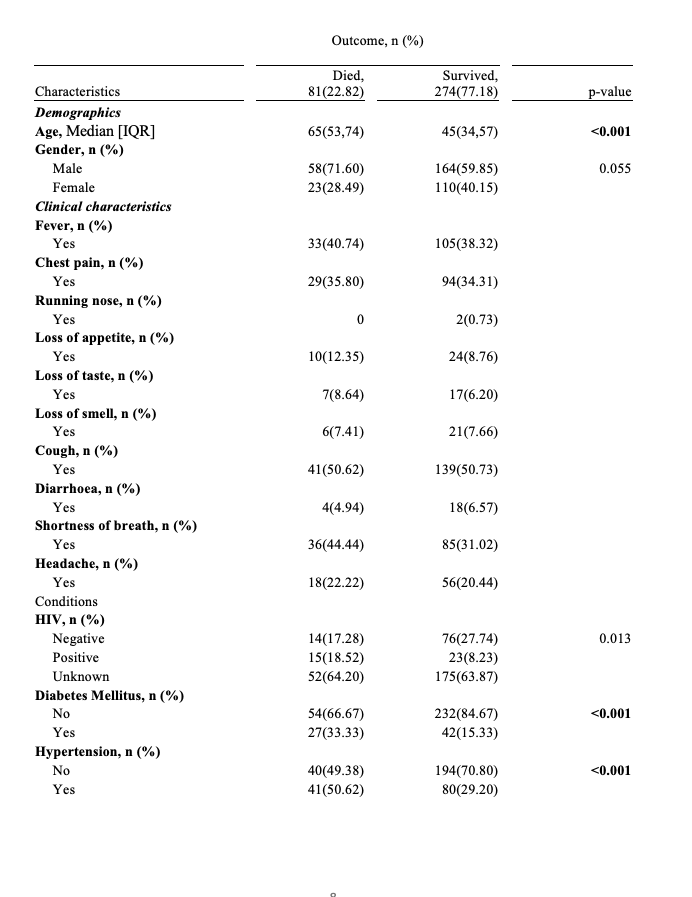
Table II below shows the relationship between outcome and demographics, clinical characteristics and underlying conditions. Majority of the Clients that died due to COVID-19 were significantly older than clients that survived (median age; 65 vs 45; p <0.001). The proportion of clients that died was higher among the male as compared to females [58 (71.60) vs 23(28.49]. Individuals with Diabetes Mellitus that died (33.33%) compared to those that survived (15.33%) was significant, p-value < 0.001 and clients who were hypertensive that died (50.62%) compared to those that survived (29.20%) was high, p- value <0.001.
Table II: Bivariate analysis of the relationship between the outcome and demographics, clinical characteristics and underlying condition
DISCUSSION
The findings depict the results of COVID-19 clients admitted at Livingstone Teaching Hospital, a period from January 2020 to September 2021. Routine screening was conducted in which demographics and clinical characteristics were recorded. Age was not normally distributed; the older population were the most affected with an increased chance of mortality [mean (48.5)]. Our findings contradicted the findings of a study done in South Korea that focused on disparities in Age specific morbidity and mortality from SARS-CoV-2 in which they observed a high morbidity and mortality to be among the young population.[12]
During the earlier phase of the COVID-19 outbreak, the diagnosis of the coronavirus was complicated by the diversity in symptoms and several clinical presentations. Our study captured the following symptoms: Cough 180 (50.7), Fever 138 (38.87%) chest pain 123(34.65%), shortness of breath 121(34.08%), headache 74(20.85%), loss of taste 24(6.67%), loss of smell 27 (7.63%), diarrhoea 22 (6.23%), running nose 2 (0.56%), loss of appetite 34 (9.58%). Gastrointestinal symptoms in COVID-19 clients admitted at Livingstone Teaching Hospital COVID-19 ward were uncommon 22(6.23%), a similar pattern in a study done by Guan and another by Jiang in China.[1] [13] However, a different pattern was observed by Wang and colleagues in which a higher proportion of cases presented with gastrointestinal symptoms including diarrhoea and nausea 14 (10%) was observed.[14] The pre-existing and co existing conditions of Diabetes mellitus and hypertension in a client proved to be a high risk factor in COVID-19 outcomes as observed. Similar findings of a previous studies conducted.[2] [15] [16]
It was observed that age was a significant risk factor of COVID-19 outcome death, most of the patients that were admitted and died were older than patients that survived (median age; 65 vs 45; p <0.001). This is illustrated in Table II that shows the relationship between COVID-19 outcome and demographics, clinical characteristics as well as underlying conditions. HIV status was a significant risk factor to COVID-19 outcome death. Our findings indicate that the percentage of clients admitted with a known HIV positive status that died were 18.52% was statistically higher than those that survived were 8.23%, p-value 0.013. These were similar conclusions derived in a study that looked at HIV infection and COVID-19 death: showed that people living with HIV had a higher risk of COVID-19 than those living without HIV after adjusting for age and sex.[17] Key to note is the inclusion of patients with unknown HIV status in the adjusted analysis, which may have diluted the true effect of confirmed HIV positivity by grouping them with those who are truly negative or by introducing noise into the model. Therefore, further research with complete HIV status ascertainment would be beneficial to fully elucidate its independent role.
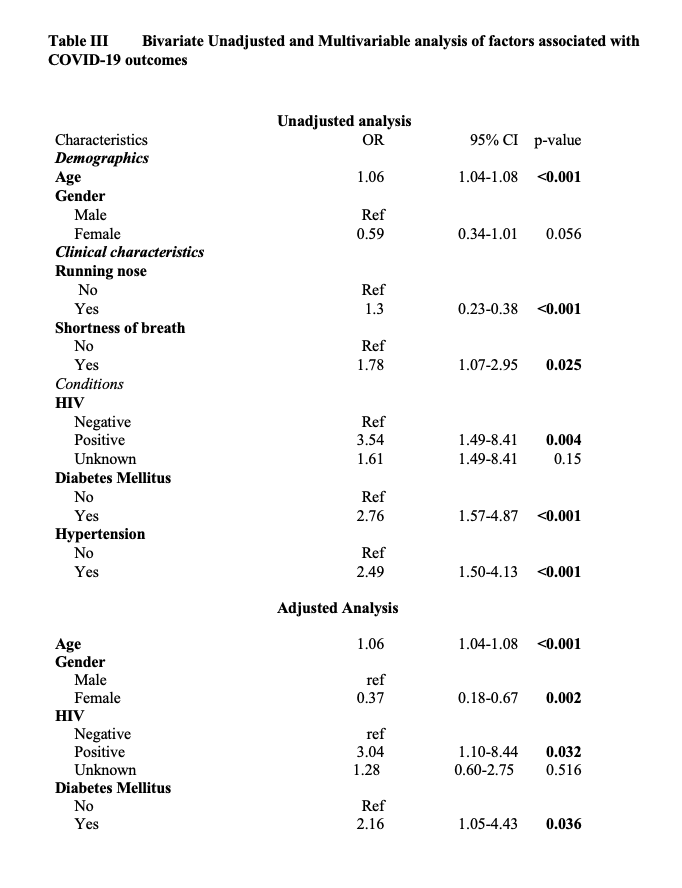
An unadjusted logistic regression (Table 4) depicts the bivariate analysis of demographics; clinical characteristics associated with COVID-19 outcomes. Being Female was a potential protection factor from the COVID-19 outcome death as they were likely to survive as compared to the male gender but is not statistically significant (OR 0.59, 95% CI 0.34-1.01; P=0.056). Similar findings in a study done on sex hormones, gender disparity in COVID-19 observed that there is a sex disparity in COVID-19 clinical outcomes, it showed that the females had a lower infection and were less likely to be hospitalized.[18] In addition, the female gender also was observed to present with a better prognosis and a less mortality rate. This trend has been observed in several studies. In China, the Chinese Centre for Disease Control and prevention reported a ratio of male to female to be 2.7:1.[19]
Evidence from the current epidemiological data has indicated a bias towards males with regards to higher susceptibility to both COVID-19 infection as well as clinical outcomes.[18] It is cardinal to note that these observations were similar to what was observed during the Severe Acute Respiratory (SARS) and Middle East Respiratory Syndrome (MERS) epidemics.20 Several factors may come to play to explain these disparity and the protection that comes with being female, this may include the intrinsic differences that exist in the innate and adaptive immune system. These differences have given the female a protective advantage therefore, exploiting such advantages can drive to developing therapeutic strategies to improve clinical outcomes.[18]
Our study included determining the association of the variables to the COVID-19 outcome. Observation made on the clinical presentation were as follows fever (OR 1.11, 95% CI 0.67-1.83; p=0.695), chest pain (OR 1.07, 95% CI 0.64-1.80; p=0.804), loss of appetite (OR 1.47, 95% CI 0.67-3.21; p=0.335), loss of taste (OR 1.43, 95% 0.57-3.58; p=0.443), cough (OR 1, 95% CI 0.61-1.63; p=0.986) and headache (OR 1.11, 95% CI 0.61-2.03; p=0.728) from these statistics generated from our study it indicated that the mentioned variables were a potential risk to COVID-19 outcomes but not a significant one. The odds ratio suggested a potential risk but however, the p value did not indicate a significance towards the outcome. This, however, does not mean these characteristics are to be overlooked. However, shortness of breath (OR 1.78, 95% CI 1.09-2.09; p=0.025) was a significant risk for the COVID-19 outcome, clients that presented with a shortness of breath upon admission had a poor prognosis. An association was established between Diabetes mellitus (OR 2.76, 95% CI 1.50-4.87; p < 0.001), Hypertension (OR 2.49, 95% CI 1.50-4.13; p < 0.001) and the outcome. These conditions were associated with a client ending up in a critical stage of COVID-19 as observed from another study that was conducted in China which observed that hypertension and Diabetes mellitus were seen to lead into a patient’s critical state.[2]
Individuals who had diabetes mellitus as an underlying condition had two-timed odd of dying (OR 2.16, 95% CI 1.05-4.43) as per our findings, it is noted that many studies have reported Diabetes to be associated with severe outcomes in terms of COVID-19 infection and mortality, however, data is conflicting. Our study has indicated a significant association. Similar findings were observed in a meta-analysis that was conducted by Kumar and colleagues to explore the relationship that existed between diabetes mellitus and COVID-19 outcomes, their results found diabetes to be significantly associated with COVID-19 mortality and a severe COVID-19 infection with a pooled ratio of 1.90 (95% CI: 1.37–2.64; p < 0.01) and 2.75 (95% CI: 2.09–3.62; p < 0.01) respectively. In this study it was indicated that Diabetes in patients with COVID-19 had a twofold increase in mortality as well as severity.[22] Another meta-analysis that looked at the prevalence and impact of diabetes among people infected with COVID-19 had similar conclusion as ours.[2]
A study was done in Italy that looked at the risk factors associated with COVID-19, their findings are similar with our observation. Hypertension was the most frequent comorbidity, and patients with hypertension had significantly decreased survival. Despite this, in the multivariable analysis, hypertension was not an independent factor associated with mortality. Our findings on the prevalence of comorbidities like hypertension and diabetes among hospitalized COVID-19 patients align with general trends observed globally, highlighting their persistent role as risk factors. Our results were also consistent with studies from other settings, which often highlight the unique interplay of co morbidities in COVID-19 outcomes. These observations resonate with Chipimo et al.'s Zambian study, which similarly documented the most prevalent underlying conditions being hypertension and human immunodeficiency virus infection whose proportion was both at 35%. This study also indicated that 20% of the patients in this study had presented with underlying condition.[23] Comparing our findings with local research like Chipimo et al. underscores both the commonalities and the specific epidemiological nuances of COVID-19 within the Zambian context, offering valuable insights for tailored public health strategies.
Beyond the clinical setting, our findings also carry significant public health implications. Given the severe cases among certain age groups and specific comorbidities, public health campaigns should prioritize targeted messaging for specific at-risk populations regarding early testing and seeking care or promoting better management of chronic diseases within the community. Furthermore, the challenges encountered in data completeness, particularly regarding HIV status, highlight the urgent need for strengthening routine health information systems at the hospital and provincial levels. Implementing standardized data collection protocols and improving the accessibility of complete patient records could significantly enhance future epidemiological surveillance and inform more effective public health interventions in Zambia.
Limitations
Majority of the clients admitted during this period were those with obvious symptoms as most were encouraged to self-isolate. Secondly, the data was only collected at a single center hospital thereby. As such the study may have included disproportionately more patients with poor outcomes.
CONCLUSION
Older age and male gender were associated with greater odds of COVID-19 infection. The main clinical manifestations of COVID-19 were cough, chest pain and shortness of breath. Our study findings highlight that older age, HIV positive status and the presence of Diabetes Mellitus are independent risk factors even after adjusting for other variables which require further investigation.
What is already known
Individuals with comorbidities are at a greater risk of COVID-19 infection and death. Hypertension and diabetic clients have a poor prognosis
What this study adds
Data on characteristics presented by COVID-19 patients generated Outcomes of COVID-19 patients from a LMC perspective
ETHICAL CONSIDERATIONS
The study received a waiver of informed consent from the Mulungushi University School of Medicine and Health Sciences Research Ethics Committee (MUSoMHS-REC) because it involved the retrospective analysis of anonymized data.
CONFLICT OF INTEREST
There was no conflict of interest
FUNDING
This research received no specific grant from any funding agency in the public, commercial or nonprofit sectors
AUTHOR CONTRIBUTIONS
- PS
- Work conception. Data acquisition, analysis and interpretation.
- Manuscript drafting.
- Approval of final manuscript.
- Accountable for all aspects of the work regarding its accuracy or integrity. MZ
- Manuscript drafting.
- Critical revisions for intellectual content.
- Data interpretation
- Approval of final manuscript.
- Accountable for all aspects of the work regarding its accuracy or integrity. PJC
- Manuscript drafting.
- Critical revisions for intellectual content.
- Data interpretation
- Approval of final manuscript.
- Accountable for all aspects of the work regarding its accuracy or integrity.
- Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. https://doi.org/10.1056/NEJMoa2002032
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13. https://doi.org/10.1016/S0140-6736(20)30211-7
- Mohamadian M, Chiti H, Shoghli A, Biglari S, Parsamanesh N, Esmaeilzadeh A. COVID‐19: virology, biology and novel laboratory diagnosis. J Gene Med. 2021;23(2):e3303. https://doi.org/10.1002/jgm.3303
- Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–54. https://doi.org/10.1038/s41579-020-00459-7 5
- Keshta AS, Mallah SI, Al Zubaidi K, Ghorab OK, Keshta MS, Alarabi D, Abousaleh MA, Salman MT, Taha OE, Zeidan AA, Elsaid MF, Tang P. COVID-19 versus SARS: A comparative review. J Infect Public Health. 2021 Jul;14(7):967-977. doi: 10.1016/j.jiph.2021.04.007. Epub 2021 Apr 24. PMID: 34130121; PMCID: PMC8064890.
- Dufailu OA, Afriyie-Asante A, Gyan B, Kwabena DA, Yeboah H, Ntiakoh F, et al. COVID-19 in Africa: an ovarian victory? J Ovarian Res. 2021;14(1):70. https://doi.org/10.1186/s13048-021-00820-1
- Massinga Loembé M, Tshangela A, Salyer SJ, Varma JK, Ouma AEO, Nkengasong JN. COVID-19 in Africa: the spread and response. Nat Med. 2020;26(7):999–1003. https://doi.org/10.1038/s41591-020-0961-x
- Simulundu E, Mupeta F, Chanda-Kapata P, Saasa N, Changula K, Muleya W, et al. First COVID-19 case in Zambia — comparative phylogenomic analyses of SARS-CoV-2 detected in African countries. Int J Infect Dis. 2021;102:455–9. https://doi.org/10.1016/j.ijid.2020.09.1480
- Baloch S, Baloch MA, Zheng T, Pei X. The coronavirus disease 2019 (COVID-19) pandemic. Tohoku J Exp Med. 2020;250(4):271–8. https://doi.org/10.1620/tjem.250.271
- Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–27.e19. https://doi.org/10.1016/j.cell.2020.06.043
- Umakanthan S, Sahu P, Ranade AV, Bukelo MM, Rao JS, Abrahao-Machado LF, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. 2020;96(1142):753–8. https://doi.org/10.1136/postgradmedj-2020-138234
- Dudley JP, Lee NT. Disparities in age-specific morbidity and mortality from SARS-CoV-2 in China and the Republic of Korea. Clin Infect Dis. 2020;71(15):863–5. https://doi.org/10.1093/cid/ciaa354
- Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med. 2020;35(5):1545–9. https://doi.org/10.1007/s11606-020-05762-w
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. https://doi.org/10.1001/jama.2020.1585
- Moon SS, Lee K, Park J, Yun S, Lee YS, Lee DS. Clinical characteristics and mortality predictors of COVID-19 patients hospitalized at nationally-designated treatment hospitals. J Korean Med Sci. 2020;35(44):e328. https://doi.org/10.3346/jkms.2020.35.e328
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–9. https://doi.org/10.1001/jama.2020.6775
- Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8(1):e24–32. https://doi.org/10.1016/S2352-3018(20)30305-2
- Raza HA, Sen P, Bhatti OA, Gupta L. Sex hormones, autoimmunity and gender disparity in COVID-19. Rheumatol Int. 2021;41(8):1375–86. https://doi.org/10.1007/s00296-021-04873-9
- Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–51. https://doi.org/10.3760/cma.j.issn.0254-6450.2020.02.003
- Alghamdi IG, Hussain II, Almalki SS, Alghamdi MS, Alghamdi MM, El-Sheemy MA. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417–23. https://doi.org/10.2147/IJGM.S67061
- Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428–55. https://doi.org/10.1111/all.14657
- Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–45. https://doi.org/10.1016/j.dsx.2020.04.044
- Chipimo PJ, Barradas DT, Kayeyi N, Zulu PM, Muzala K, Mazaba ML, et al. First 100 persons with COVID-19 – Zambia, March 18–April 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1547–8. https://doi.org/10.15585/mmwr.mm6942a5
ACKNOWLEDGEMENT
This article is a part of the master thesis submitted to the University of Lusaka in partial fulfilment of the requirements for the degree of Master of Public Health in respect of Ms Precious Simushi.
REFERENCES
Medical Journal of Zambia, Vol 52, 5
The Medical Journal of Zambia, ISSN 0047-651X, is published by the Zambia Medical Association.
© This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.