Comparative Evaluation of GeneXpert and Chest X-ray in Diagnosing Paediatric Tuberculosis at Arthur Davison Children's Hospital, Zambia
David Chisompola
Arthur Davison Children's Hospital
Prisca Chanda
Arthur Davison Children's Hospital
Roy Moono
Arthur Davison Children's Hospital
Moses Chakopo
Arthur Davison Children's Hospital
Patrick Chipengwe
Arthur Davison Children's Hospital
DOI: https://doi.org/10.55320/mjz.52.2.643
Keywords:Tuberculosis; diagnosis; children; GeneXpert; chest X-ray; Zambia.
ABSTRACT
Introduction
Tuberculosis (TB) remains a major global health issue, affecting 10-20% of children worldwide. Zambia is one of the 30 high-burden TB countries. Diagnosing Mycobacterium tuberculosis in children is particularly challenging. Early detection and treatment are crucial for effective management and preventing severe disease progression. This study aimed to assess TB prevalence in children at Arthur Davison Children's Hospital in Ndola, Zambia, using GeneXpert on gastric lavage samples and X-ray imaging.Method
This was a cross-sectional study conducted at Arthur Davison Children’s Hospital from October 2021 to February 2022. Children aged 0 to 10 years with suspected presumptive tuberculosis were enrolled for diagnostic evaluation. Tuberculosis diagnosis was performed using the GeneXpert polymerase chain reaction analyzer on gastric lavage samples, compared with findings from chest X-ray imaging. Data analysis was carried out using descriptive statistics in the Statistical Package for Social Sciences (SPSS) version 22, with statistical significance set at p < 0.05.
Results
Out of the 138 participants enrolled, the prevalence of tuberculosis was 13.8% based on X-ray imaging and 1.4% using the GeneXpert analyzer. Males had a higher prevalence of tuberculosis (52.6%) compared to females (47.4%). The age group of participants who were mostly affected was 0-4 years (68.4%). The agreement between the two diagnostic methods, measured by Cohen's Kappa Coefficient, was 0, indicating no agreement. The p-value for the comparison was 1.19.Conclusion
The findings of this study highlight significant presence of tuberculosis among children under 10 years of age at Arthur Davison Children’s Hospital. However, there was no agreement between the diagnostic methods used, namely GeneXpert and X-ray imaging. Further comprehensive studies are recommended to investigate the potential for false positives associated with X-ray imaging or false negatives on the GeneXpert analyzer.
INTRODUCTION
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), remains one of the leading causes of death worldwide from a single infectious agent.[1] After three years of being surpassed by coronavirus disease (COVID-19),[2] TB has likely regained this position and caused almost twice as many deaths as HIV/AIDS.[2] More than 10 million people continue to fall ill with TB every year and the number has been rising since 2021. TB in Zambia is one of the leading causes of morbidity and mortality[3] especially with the heavy HIV/AIDS burden in the region.[4] TB incidence declined from 759 per 100,000 in 2000 to 319 per 100,000 in 2020.[5] In 2021, Zambia reported 50,007 TB cases, with 6-7.8% (3,890) among children aged 0-14 years.[6] By 2022, the situation showed improvement, with 68% of estimated paediatric TB cases diagnosed and treated. However, these figures highlight persistent gaps in the care of children with TB, emphasizing the urgent need for enhanced diagnostic tools, targeted interventions, and improved reporting mechanisms to address this vulnerable population.
Rapid diagnosis of TB remains a significant challenge, particularly in paediatric populations, as the gold standard culture method is time consuming (2-8 weeks),[7] has variable yield, and requires specialized laboratory infrastructure, which is limited in Zambia. Molecular tools like GeneXpert, which detect TB and rifampicin resistance rapidly, have been introduced, but their sensitivity in paediatric using gastric lavage samples remains suboptimal and is often necessary due to difficulties in obtaining sputum samples. While chest X-rays (CXRs) are sensitive for TB diagnosis,[8] they cannot detect drug resistance, limiting their utility in guiding treatment. Despite recommendations by the Zambia National Tuberculosis and Leprosy Control Program to use GeneXpert as the initial test for paediatric TB, with CXR as a follow-up,[9] there is a critical lack of research evaluating the effectiveness, accessibility, and applicability of these TB diagnostic tools in Zambia's resource-limited settings. Addressing this gap is essential to improving early diagnosis, reducing TB-related morbidity and mortality, and achieving national and global TB control targets, particularly given the high burden of TB among vulnerable paediatric populations in the region.
The GeneXpert MTB/RIF assay demonstrates superior sensitivity and accuracy compared to Acid-Fast Bacilli (AFB) smear and Mycobacteria Growth Indicator Tube (MGIT) culture for diagnosing pulmonary TB, particularly in smear-negative children and non-expectorating adults.[10] While it enhances TB detection in gastric lavage samples compared to culture, its sensitivity in young children remains lower than MGIT culture, with no significant difference between induced sputum and gastric lavage. Studies by Khanam et al. and Tahmida et al. report that the sensitivity of MGIT and GeneXpert in detecting clinically diagnosed TB in children was 11.67% and 13.1%,[11][12] respectively, using gastric aspirates, highlighting the challenges in paediatric TB diagnosis. However, GeneXpert performs well in bronchoalveolar lavage samples, underscoring its value for early and accurate diagnosis.[13] In contrast, while chest X-ray (CXR) is highly sensitive for detecting pulmonary TB and can reveal TB-specific abnormalities such as cavities, its low specificity and variability in interpretation among observers limit its diagnostic utility, often leading to overdiagnosis or underdiagnosis.[14] Consequently, bacteriological confirmation through sputum smear microscopy, culture, or molecular testing remains essential for accurate TB diagnosis, particularly in resource-limited settings where diagnostic challenges are compounded.
In this study, we aimed to compare the GeneXpert and Chest X-ray in Diagnosing Paediatric Tuberculosis at Arthur Davison Children's Hospital (ADCH), Zambia. By comparing these diagnostic tools, we sought to provide a baseline analytical performance, bridge gaps in paediatric TB detection, and support national TB control efforts, ultimately enhancing early diagnosis, and reducing TB-related morbidity and mortality in Zambian children.
METHODS
This was a cross-sectional study performed at ADCH in Ndola, Zambia from October 2021 to February 2022. ADCH is a specialized and main referral Children's Hospital covering all paediatric patients from 0 to 14 years in the northern part of Zambia.
Inclusion and exclusion criteriaChildren aged 10 years or younger presenting with symptoms suggestive of Mycobacterium tuberculosis infection were included in the study if their guardians provided informed consent and assent. Eligibility was confirmed through a diagnostic evaluation using both GeneXpert testing and chest X-ray imaging.[9] Children were excluded if their guardians did not provide consent or assent, or if only one of the two diagnostic methods (GeneXpert or X-ray imaging) was used for diagnosis.
Sample SizeGiven that the prevalence of TB in Zambia is well-documented, with the first national TB prevalence survey revealing a bacteriologically confirmed MTB prevalence of approximately 69 cases per 100,000 children, this translates to an estimated prevalence of 0.1%.[15] Based on this prevalence, the sample size was calculated as follows:
N=(Z² p(1-p))/e²
N=((1.96)² (0.1)(1-0.1))/0.05²
N=138.2976
A total of 138 samples were included in the study.
Sampling techniqueConvenience sampling was used to enroll participants, focusing on children with suspected TB symptoms who were readily accessible at ADCH during the study period. While this approach facilitated efficient participant recruitment, it may limit the application of the findings to the broader paediatric population, as the sample may not fully represent all children with TB in Zambia, particularly those who do not access healthcare services. Furthermore, the study may be subject to selection bias, as it focused exclusively on patients within a specific age group who sought treatment at ADCH and included only those who were available during the study period.
Sample processing and ImagingThe GeneXpert MTB/RIF assay, a nucleic acid amplification test, was performed using the GeneXpert Dx 4.7b Software System and disposable cartridges (Cepheid, Sunnyvale, California, USA).[16] This system is globally recognized for diagnosing infectious diseases, including tuberculosis (TB).[17] The GeneXpert MTB/RIF Ultra analyzer was employed for diagnosing TB from gastric lavage samples, following the Centre for Disease Control and Prevention (CDC) guidelines[18] and following the manufacturer's protocol targeting the rpoB gene. Briefly, 0.5 mL of each gastric lavage sample was mixed with 1.5 mL of GeneXpert reagent, incubated at room temperature for 15 minutes, and mixed adequately at 10 and 15 minutes. Next, 2 mL of the diluted sample was transferred to a GeneXpert MTB/RIF cartridge and processed using the GeneXpert IV instrument. Results were interpreted using GeneXpert software, with MTB positivity load reported semi quantitatively: very low (Ct > 28), low (Ct 22-28), medium (Ct 16-22), or high (Ct < 16).[19]
Gastric lavage samples were collected by a trained nurse and transported to the laboratory for processing by qualified laboratory technologists and scientists. To ensure participant confidentiality, samples were de-identified using unique study codes. Sample preparation and processing adhered strictly to the manufacturer's instructions, utilizing the provided reagents.
Participants also underwent chest X-ray imaging, performed by a trained radiologist. GeneXpert MTB results were not availed to the radiologists. All personal information was kept confidential and used exclusively for research purposes. A trained radiologist interpreted the X-ray results.
Bias minimization strategiesTo minimize bias in the comparison of diagnostic methods, several strategies were implemented. First, samples sent to the laboratory for GeneXpert testing and to the radiology department for chest X-ray imaging were blinded, ensuring that technicians and radiologists were unaware of the results from the other method. Only the Principal Investigator had access to the results for analysis. Second, participants were not given special treatment; they were processed routinely alongside other patients in the laboratory and radiology departments to maintain consistency and avoid preferential handling. Additionally, to ensure accuracy and reliability in imaging interpretation, two independent radiologists reviewed the chest X-ray results. In cases of disagreement, a third radiologist was consulted to reach a consensus. These measures were designed to reduce diagnostic bias and enhance the validity of the findings.
Data CollectionData was collected using a structured Excel spreadsheet, capturing demographic information (age and sex) and diagnostic results from both X-ray imaging and the GeneXpert MTB/RIF assay. Parents and/or guardians of enrolled participants completed a structured questionnaire and provided informed consent and assent.
Data AnalysisData from the Excel spreadsheet was imported into the Statistical Package for Social Sciences (SPSS) version 22 for analysis. Descriptive statistics were used to summarize the data, with gender proportions expressed as percentages and age categorized into two groups: 0-4 years and 5-10 years. Agreement between diagnostic methods was assessed using Cohen's Kappa Coefficient, and McNemar's test with statistical significance set at p < 0.05.
Ethics approval and consent to participateEthical approval was obtained from the University of Zambia School of Medicine Undergraduate Research Ethics Committee (IRB: 00001131, FWA: 000000338; reference number 27-07-2022) and the National Health Research Authority (18th July 2022). Permission to conduct the study was granted by ADCH management. Written informed consent and assent were obtained from parents and/or guardians prior to participation. To ensure confidentiality and anonymity, each participant was assigned a unique project identification number, used exclusively for research purposes. All personal identifiers were removed from the data, and access to participant information was restricted to authorized research personnel only. Data were stored securely in password-protected electronic files and locked cabinets, with regular backups to prevent loss. These measures were implemented to comply with ethical standards and ensure the protection of participant confidentiality and data integrity throughout the study.
RESULTS
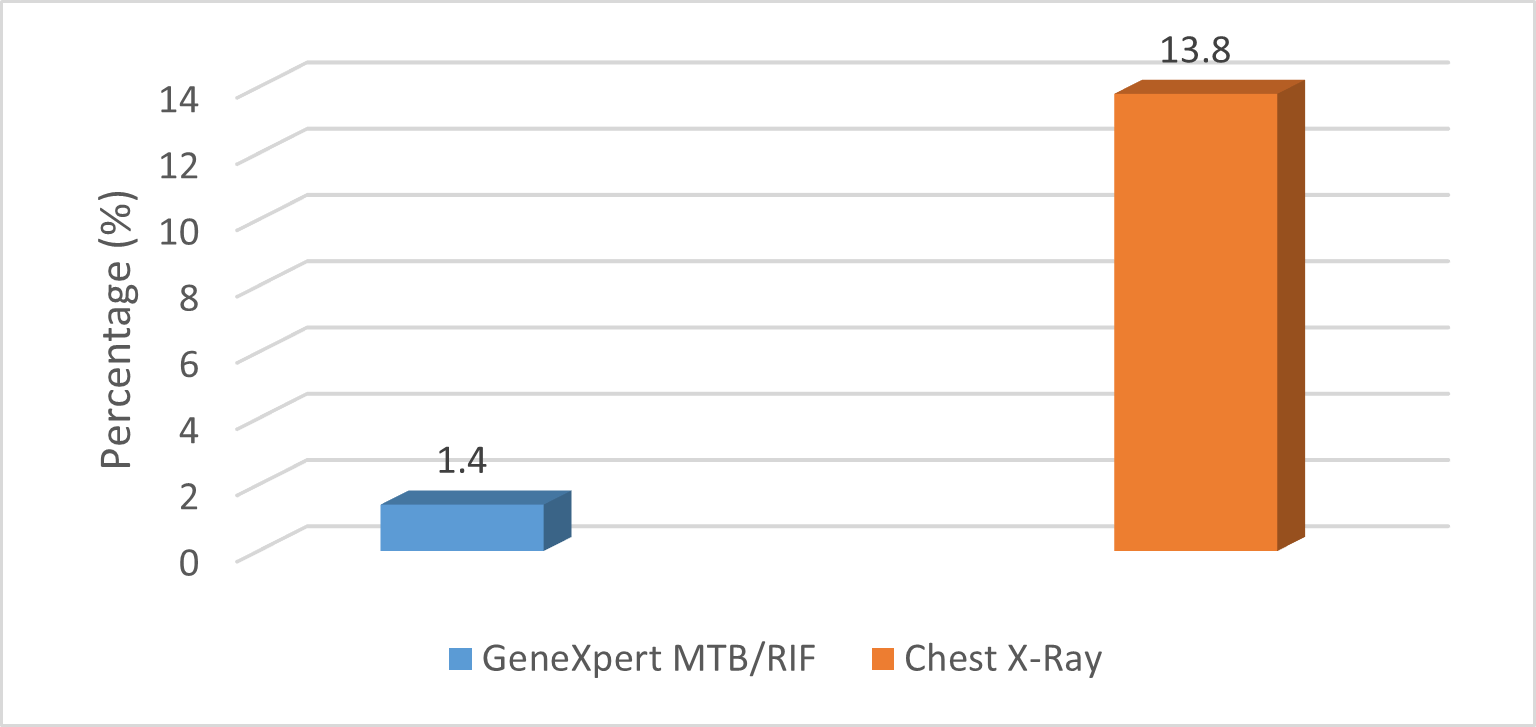
A total of 138 participants were included in the study. The Detection of TB varied depending on the diagnostic method used. X-ray imaging identified 19 cases 13.8% (95% CI: 8.2-20.7), whereas GeneXpert confirmed only 2 cases 1.4% (95% CI: 0.2-5.1) Table1. The gender distribution among the TB-positive cases was nearly equal, with 10 males 52.6% (95% CI: 31.71-72.67) and 9 females 47.4% (95% CI: 27.33-68.29). Regarding age groups, most TB-positive participants were aged 0-4 years 68.4% (95% CI: 46.0 -84.64), while 31.6% (95% CI: 15.36-53.99) were in the 5-10 years age as shown in Table1.
Table 1. Detection of Tuberculosis
| Variable | Category | Frequency (n) | Percentage (%) | 95% CI |
|---|---|---|---|---|
| Total Participants | - | 138 | 100.0 | - |
| X-ray Imaging | 19 | 13.8 | 8.2-20.7 | |
| TB Detection | GeneXpert Using Gastric lavage | 2 | 1.4 | 0.2-5.1 |
| Gender Distribution | Male | 10 | 52.6 | 31.71 - 72.67 |
| Female | 9 | 47.4 | 27.33 - 68.29 | |
| Age Group (years) | 0 - 4 | 13 | 68.4 | 46.01 - 84.64 |
| 5 - 10 | 6 | 31.6 | 15.36 - 53.99 |
Comparison between GeneXpert MTB/RIF and Chest X-ray imaging
The agreement between X-ray imaging and GeneXpert, assessed using Cohen's Kappa coefficient, was 0, (95% CI: -0.05 to 0.05) indicating no agreement between the two diagnostic methods. In this context, the complete lack of agreement between X-ray imaging and GeneXpert MTB/RIF suggests that the two methods produce entirely inconsistent results when diagnosing TB. Additionally, the McNemar's test yielded a chi-square statistic of χ² = 17.0 (95% CI: 29.4%-70.6%), with a p-value of 3.7 × 10⁻⁵. Given that the p-value is significantly lower than the 0.05 threshold, we reject the null hypothesis, indicating a statistically significant difference in TB detection between the two methods. Specifically, Chest X-ray imaging identified significantly more TB cases than GeneXpert MTB/RIF, suggesting a potential difference in sensitivity between these diagnostic approaches. As Shown in table 2.
Table 2. Comparison between GeneXpert MTB/RIF and Chest X-ray imaging
| Method | Frequency (n=138) | Percentage (%) | 95% CI | p Value | McNemar's test | Cohen's Kappa Coefficient |
|---|---|---|---|---|---|---|
| X-ray Imaging | 19 | 13.8 | 8.2-20.7 | 3.7 × 10⁻⁵ | 17 | 0 |
| GeneXpert Using Gastric lavage | 2 | 1.4 | 0.2-5.1 |
Detection of Tuberculosis by X-ray imaging identified 19 cases (13.8%; 95% CI: 8.2-20.7), while GeneXpert confirmed only 2 cases (1.4%; 95% CI: 0.2-5.1). As shown in figure 1.
Figure 1. Tuberculosis Detection by Method
Detection of Tuberculosis by Age Groups: Among TB-positive participants, the majority 68.4% (95% CI: 46.0 -84.64) were aged 0-4 years, while the remaining 31.6% (95% CI: 15.36-53.99) were in the 5-10 years age group. As shown in figure 2.
Figure 2. Tuberculosis Detection by Age
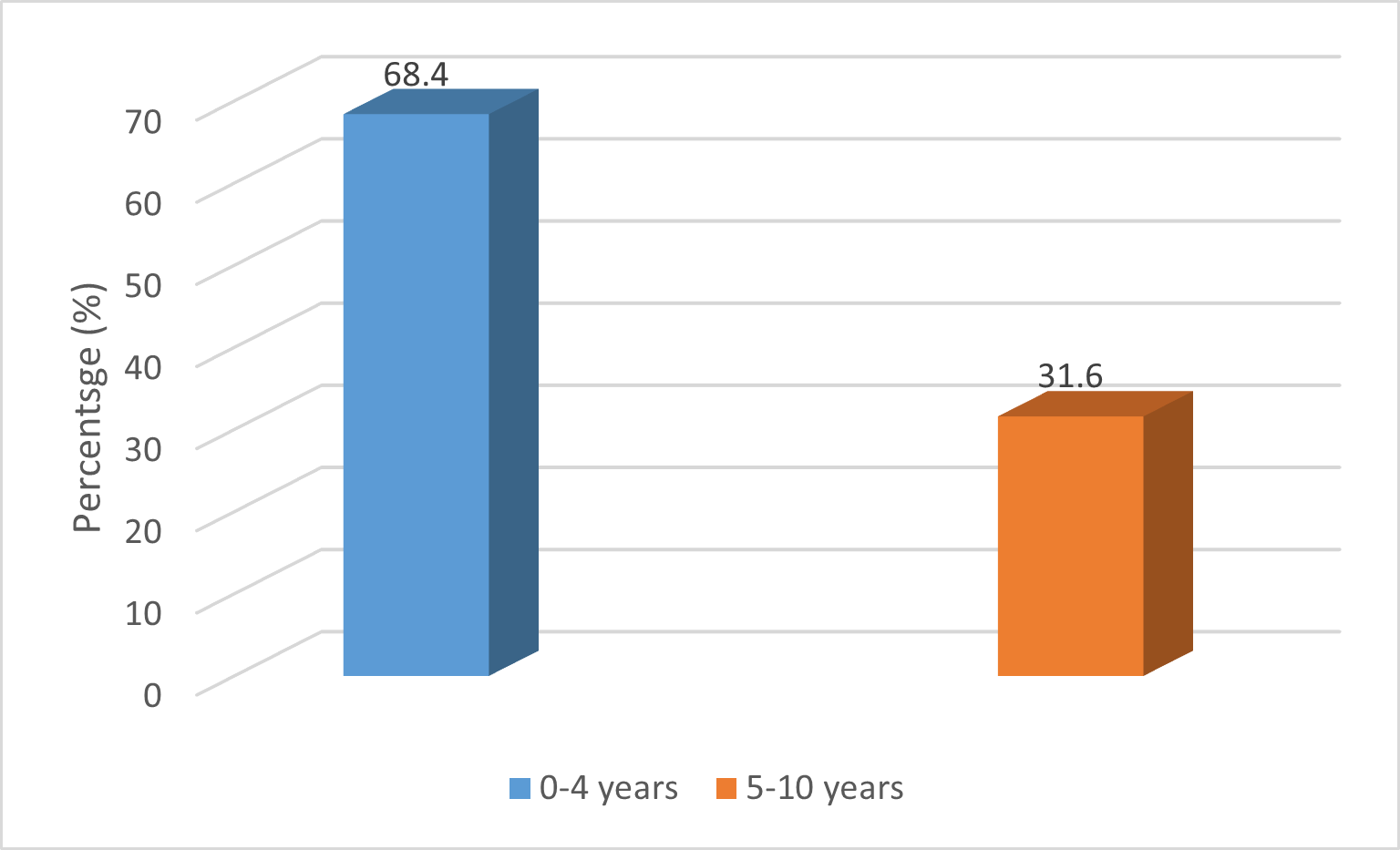
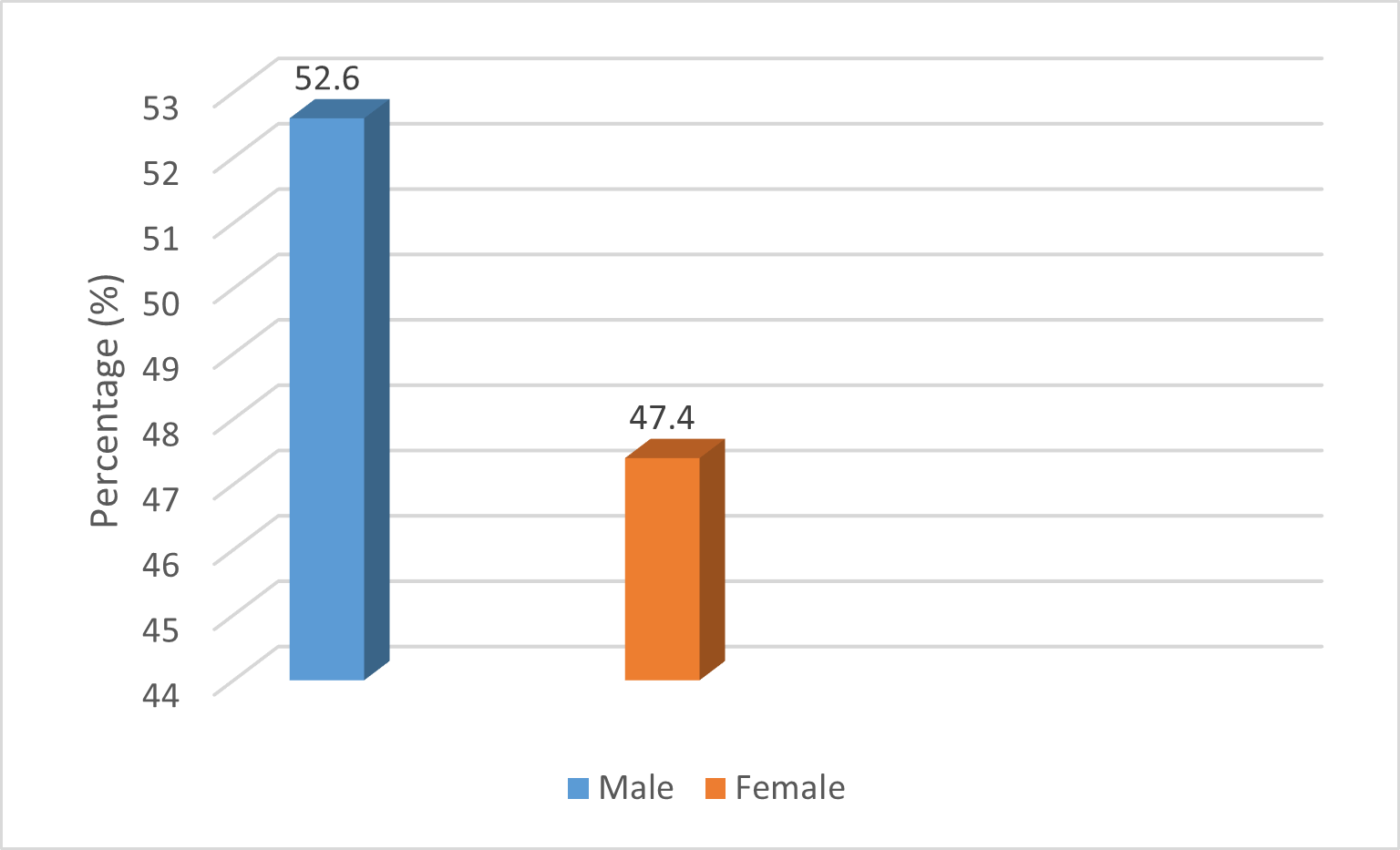
Detection of Tuberculosis by gender showed a nearly equal distribution among TB-positive cases, with 10 males 52.6% (95% CI: 31.71-72.67) and 9 females 47.4% (95% CI: 27.33-68.29). As shown in figure 3.
Figure 3. Tuberculosis Detection by Sex
DISCUSSION
This study compared the detection of TB using Chest X-ray imaging and GeneXpert MTB/RIF in paediatrics attending ADCH in Zambia. To the best of the authors' knowledge, this is the first study of its kind conducted on paediatric patients to evaluate the analytical performance of diagnostic tools for TB in our setting. Our study findings showed a significant difference in detection rates, with Chest X-ray identifying 19 cases (13.8%) while GeneXpert confirmed only 2 cases (1.4%). The McNemar's test (χ² = 17.0, p = 3.7 × 10⁻⁵, 95% CI: 29.4%-70.6%) confirmed this difference as statistically significant. Additionally, Cohen's Kappa coefficient was 0 (95% CI: -0.05 to 0.05), indicating no agreement between the two methods. These findings highlight important differences in the sensitivity and diagnostic consistency of the two methods, with implications for TB case detection and management.
The GeneXpert MTB/RIF detection in gastric lavage samples aligns with the 1.3% reported by Dindi et al[20] in Zambia. However, it is notably lower than the detection rates reported in Nepal by Roma et al, 5.1%,[21] Tahira Nishtar et al 8.7%,[22] and in Zambia 1.58%.[23] These differences highlight the variability in the performance of the GeneXpert MTB/RIF assay in detecting TB across different settings. This variability may be attributed to the presence of gastric acid, which neutralizes samples and kills microorganisms, as well as factors such as improper sample collection, transportation, storage, and decontamination.[24]
The TB detection rate using CXR in our study was lower than the 16% reported at a tertiary children's Hospital in the United Kingdom.[25] Conversely, our study reported a higher TB detection rate compared to the 5.1% reported by Garcia et al. in Mozambique[26] and the 3.6% reported by Lozano et al. in Colombia.[27] Variations in TB detection may be attributed to differences in diagnostic screening strategies, access to healthcare services, and the availability of diagnostic tools for childhood TB diagnosis and differences in sample size used.
There was limited literature available, particularly in our setting, to contextualize our findings and assess the diagnostic accuracy of tools such as CXR imaging and the GeneXpert MTB/RIF assay. Therefore, literature from diagnostic evaluations of CXR and GeneXpert MTB/RIF in paediatric populations from other settings and regions was utilized, as these findings provide relevant insights into the accuracy and performance of imaging examinations.
Our findings also revealed a slightly higher tuberculosis detection rate in males (52.6%) compared to females (47.4%). The detection rate by gender in our study was nearly equal. However, differences in gender detection have been observed in other studies, which may be attributed to variations in study design and sample size.[28] Additionally, children aged 0 to 4 years were the most affected group, with a detection rate of 68.4%, while 31.6% of cases were detected in children aged 5 to 10 years. This age-related difference may be attributed to variations in exposure and immune response between the two groups.[29] Furthermore, Zanozin et al. highlights that paediatrics, particularly those under three years of age, are at a higher risk for rapid progression to active TB due to their immature immune systems.[29]
Our findings demonstrated no agreement between the two diagnostic methods, as indicated by Cohen's Kappa Coefficient of 0 and a p-value of 3.7 × 10⁻⁵. This result supports the rejection of the null hypothesis, which stated that there is no significant difference in the diagnostic performance of GeneXpert and Chest X-ray in detecting paediatric tuberculosis at Arthur Davison Children's Hospital, Zambia. Sorsa reported a higher Cohen's Kappa Coefficient of 0.38, highlighting the variability in agreement between diagnostic methods across different studies.[30] Similarly, Wekesa et al. observed a high rate of false positives with CXR, where 42.5% of patients had CXR results suggestive of TB, yet only 17% tested positive using GeneXpert, which was considered the gold standard.[31] This discrepancy in the results indicates that CXR may lead to an overestimation of TB cases, leading to unnecessary treatment or additional investigations for those who do not have active TB. Clinical and radiological features of TB can mimic those of many other diseases, making it essential to employ additional diagnostic strategies, such as culture and biopsy, for a definitive diagnosis.[32][33][34] Furthermore, COVID-19 infections have contributed to numerous false-positive TB results on CXR due to similar lung abnormalities, complicating differentiation between the two conditions. Radiological diagnosis in children is particularly challenging due to the absence of classic radiological findings typically indicative of TB.[32]
These findings have important implications for TB screening strategies. The significantly higher detection rate of Chest X-ray suggests that molecular tests alone may underestimate TB detection, particularly in settings with high paediatric TB burden or in individuals with subclinical infections. However, the lack of specificity in Chest X-ray findings could lead to overdiagnosis if not confirmed by microbiological tests. Integrating Chest X-ray as a screening tool with follow-up molecular or culture-based confirmation may improve TB case detection while minimizing false positives.
A key strength of this study are the statistical tools applied, including McNemar's test and Cohen's Kappa analysis, which provide robust evidence of the disagreement between the two methods. However, the lack of a gold standard reference method (such as TB culture or AFB smear) limits the ability to determine which method is more accurate. Additionally, the small sample size may affect the generalizability of findings. Future studies should incorporate culture-based confirmation and larger cohorts to validate these results. Additionally, there's need to explore the cost-effectiveness of combining Chest X-ray imaging and GeneXpert MTB/RIF, as well as the role of clinical context and patient demographics in diagnostic accuracy. Finally, studies should also investigate the impact of these diagnostic strategies on treatment outcomes and TB transmission in endemic regions. We did not account for the clinical characteristics of participants, such as HIV/AIDS status and Bacillus Calmette-Guérin (BCG) vaccination history. Further, the study was conducted during the COVID-19 pandemic, which may have contributed to an overestimation of TB detection using CXR.
Limitations
The study was conducted over a six-month period at a single Hospital, Arthur Davison Children's Hospital. This limited scope may not fully capture the overall detection rate of TB or the broader diagnostic challenges in paediatric populations across Zambia. Additionally, the study may be subject to selection bias, as it focused solely on patients within a specific age group who sought treatment at Arthur Davison Children's Hospital. This could lead to an overrepresentation of more severe cases while excluding certain subpopulations, potentially affecting the accuracy and generalizability of the findings.
CONCLUSION
This study demonstrates a significant difference in TB detection between Chest X-ray imaging and GeneXpert MTB/RIF, with Chest X-ray identifying more cases. However, the lack of agreement between the two methods suggests that neither should be used in isolation for TB diagnosis. Diagnosing TB in children remains challenging due to their inability to produce sputum. Instead, gastric lavage is often used as a diagnostic method, but it frequently yields a low TB detection rate. The study underscores the importance of using multiple diagnostic methods to improve the accuracy of TB diagnosis in children. While Chest X-ray imaging remains a valuable tool, its limitations highlight the need for complementary methods like GeneXpert MTB/RIF, especially in high-burden regions like Zambia. Developing targeted and efficient TB detection strategies will require a deeper understanding of the strengths and weaknesses of each diagnostic approach. A larger study is needed to validate these findings, assess diagnostic discrepancies, and refine childhood TB detection guidelines. Future research should focus on evaluating combined diagnostic approaches and assessing their impact on TB case detection and treatment outcomes.
RecommendationsBased on the study's findings, several recommendations have been proposed for TB screening in paediatric patients in Zambia. Screening should begin with a highly sensitive test such as CXR, followed by microbiological confirmation using GeneXpert MTB/RIF, culture, or AFB smear. However, CXR should not be used in isolation, as it cannot detect drug-resistant TB, including rifampicin and isoniazid resistance. A combined approach using GeneXpert and clinical assessments should be prioritized to prevent unnecessary treatments especially in diseases that resemble TB in CXR such as Covid-19. Given the higher detection rate of TB in children aged 0 to 4 years, targeted screening and early detection should be strengthened for this high-risk group. Healthcare professionals need training on the limitations of various TB diagnostic methods to ensure accurate clinical decisions. Expanding research beyond a single hospital and incorporating community-based screening will improve surveillance, minimize selection bias, and strengthen early diagnosis and treatment outcomes in high-burden regions like Zambia.
What is already known on this topic:Children face a heightened risk of developing severe forms of TB, such as TB meningitis and miliary TB, particularly if they are under five years of age or living with HIV. Diagnosing TB in children presents significant challenges due to non-specific symptoms, the difficulty of collecting sputum samples, and the frequently TB paucibacillary nature of the disease.
What this study adds:This study evaluates diagnostic methods for Tuberculosis detection in children, focusing on overcoming the challenges of routine sputum-based diagnosis. Due to children's limited ability to produce sputum, the study explores alternative approaches, such as gastric lavage samples analyzed with GeneXpert and X-ray imaging, to improve Tuberculosis detection and management in paediatric populations.
AcknowledgementsWe sincerely acknowledge the staff of the Microbiology Laboratory, the Hospital management at Arthur Davison Children's Hospital, and the Provincial Health Office, Ministry of Health, Copperbelt Province, for their support and for granting us the necessary authorization to conduct this study in the Laboratory Department.
Competing interestsThe authors declare that they have no competing interests.
Authors' contributionsPriscah Chanda conceived the study. Roy Moono and Patrick Chipengwe conducted data collection. David Chisompola and Priscah Chanda conducted data analysis and writing of the final manuscript. Moses Chakopo reviewed. All authors read and approved of the final manuscript.
REFERENCES
- Cegielski JP, Dalton T, Yagui M, Wattanaamornkiet W, Volchenkov GV, Via LE, et al. Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin Infect Dis [Internet]. 2014 Oct 15;59(8):1049–63. Available from: https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/ciu572
- World Health Organization. 2024 Global Tuberculosis Report. Geneva: WHO; 2024.
- Lungu P, Kerkhoff AD, Kasapo CC, Mzyece J, Nyimbili S, Chimzizi R, et al. Tuberculosis care cascade in Zambia - identifying the gaps in order to improve outcomes: a population-based analysis. BMJ Open [Internet]. 2021 Aug 10;11(8):e044867. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34376439
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Fact sheet 2024 - Latest global and regional HIV statistics on the status of the AIDS epidemic. 2024. Available from: https://www.unaids.org/en
- Ngosa D, Lupenga J. Childhood tuberculosis outcomes and factors associated with unsuccessful treatment outcomes in selected public hospitals of Lusaka, Zambia from 2015 to 2019. PLoS Glob Public Health [Internet]. 2024 Oct 11;4(10):e0002591. Available from: https://dx.plos.org/10.1371/journal.pgph.0002591
- Kagujje M, Nyangu S, Maimbolwa MM, Shuma B, Mutti L, Somwe P, et al. Strategies to increase childhood tuberculosis case detection at the primary health care level: Lessons from an active case-finding study in Zambia. PLoS One [Internet]. 2023 Jul 19;18(7):e0288643. Available from: https://dx.plos.org/10.1371/journal.pone.0288643
- Diktanas S, Korotych O, Sereda Y, Gozalov O, Rubcova O, Achar J. Factors associated with time to sputum culture conversion of rifampicin-resistant tuberculosis patients in Klaipeda, Lithuania (2016–2019): a cohort study. Monaldi Arch Chest Dis [Internet]. 2021 Jan 14;91(1). Available from: https://www.monaldi-archives.org/index.php/macd/article/view/1675
- Crowder R, Thangakunam B, Andama A, Christopher DJ, Dalay V, Dube-Nwamba W, et al. Head-to-head comparison of diagnostic accuracy of TB screening tests: Chest X-ray, Xpert TB host response, and C-reactive protein [Internet]. 2024. Available from: http://medrxiv.org/lookup/doi/10.1101/2024.06.20.24308402
- Zambia Ministry of Health. The National Tuberculosis and Leprosy Control Program [Internet]. 2019. p. 525–6. Available from: https://www.afro.who.int/publications/guidelines-programmatic-management-drug-resistant-tb-zambia-third-edition
- Ghafoor L, Zuberi FF, Khan GM, Ismail M. Yield and accuracy of gastric lavage in non-expectorating adults with suspected pulmonary tuberculosis. Pak J Med Sci [Internet]. 2023 Jul 10;39(5). Available from: https://www.pjms.org.pk/index.php/pjms/article/view/6972
- Khanam M, Rahat F, Hossain B, Abiduzzaman M, Ghosh NK. Diagnosis of pulmonary tuberculosis by Xpert MTB/RIF assay using gastric aspirate in children with pneumonic consolidation. Bangladesh J Child Health [Internet]. 2024 May 7;46(2):65–70. Available from: https://www.banglajol.info/index.php/BJCH/article/view/72113
- Haque T, Hossain D, Islam T. GeneXpert Ultra - A new diagnostic era for childhood tuberculosis. Sch Acad J Biosci [Internet]. 2022 Nov 1;10(11):266–72. Available from: https://saspublishers.com/media/articles/SAJB_1011_266-272_c.pdf
- Soo CI, Lim KY, Rajah R, Ng BH, Abdul Hamid F, Ban AYL. Comparison of utilizing GeneXpert MTB/RIF and other conventional methods on bronchoalveolar lavage in the diagnosis of Mycobacterium tuberculosis: A real-life retrospective study. Tuberculosis [Internet]. 2019. p. PA552. Available from: http://publications.ersnet.org/lookup/doi/10.1183/13993003.congress-2019.PA552
- Toyoda Y, Nakayama T, Kusunoki Y, Iso H, Suzuki T. Sensitivity and specificity of lung cancer screening using chest low-dose computed tomography. Br J Cancer [Internet]. 2008 May 6;98(10):1602–7. Available from: https://www.nature.com/articles/6604351
- Kapata N, Chanda-Kapata P, Ngosa W, Metitiri M, Klinkenberg E, Kalisvaart N, et al. The prevalence of tuberculosis in Zambia: Results from the first national TB prevalence survey (2013–2014). PLoS One [Internet]. 2016;11(1):e0146392. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26771588
- Dahiya B, Mehta N, Soni A, Mehta PK. Diagnosis of extrapulmonary tuberculosis by GeneXpert MTB/RIF Ultra assay. Expert Rev Mol Diagn [Internet]. 2023 Jul 3;23(7):561–82. Available from: https://www.tandfonline.com/doi/full/10.1080/14737159.2023.2223980
- Lawn SD, Mwaba P, Bates M, Piatek A, Alexander H, Marais BJ, et al. Advances in tuberculosis diagnostics: The Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis [Internet]. 2013 Apr;13(4):349–61. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1473309913700082
- Pang Y, Wang Y, Zhao S, Liu J, Zhao Y, Li H. Evaluation of the Xpert MTB/RIF assay in gastric lavage aspirates for diagnosis of smear-negative childhood pulmonary tuberculosis. Pediatr Infect Dis J [Internet]. 2014 Oct;33(10):1047–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25361186
- Fradejas I, Ontañón B, Muñoz-Gallego I, Ramírez-Vela MJ, López-Roa P. The value of Xpert MTB/RIF-generated CT values for predicting the smear status of patients with pulmonary tuberculosis. J Clin Tuberc Other Mycobact Dis. 2018 Dec;13:9–12. Available from: https://pubmed.ncbi.nlm.nih.gov/31720405/
- Dindi M, Chabala C, Somwe S. Prevalence of tuberculosis in HIV- or tuberculosis-exposed neonates at the University Teaching Hospital in Lusaka over six months. Med J Zambia [Internet]. 2023 Nov 15;49(4). Available from: https://mjz.co.zm/index.php/mjz/article/view/390
- Roma K, Shrestha SK, Bhandari N, Shah GJ, Khetan S. Assessment of GeneXpert test for diagnosis of pediatric pulmonary tuberculosis. Birat J Health Sci [Internet]. 2023 Nov 27;8(2):2024–8. Available from: https://www.nepjol.info/index.php/bjhs/article/view/59853
- Nishtar T, Burki S, Ahmad FS, Ahmad T. Diagnostic accuracy of computer-aided reading of chest X-ray in screening for pulmonary tuberculosis in comparison with GeneXpert. Pak J Med Sci [Internet]. 2021 Nov 20;38(1). Available from: http://pjms.org.pk/index.php/pjms/article/view/4531
- Munthali T, Chabala C, Chama E, Mugode R, Kapata N, Musonda P, et al. Tuberculosis caseload in children with severe acute malnutrition related to high hospital-based mortality in Lusaka, Zambia. BMC Res Notes [Internet]. 2017 Dec 12;10(1):206. Available from: http://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-017-2529-5
- Tan HK, Fan SJ, Xu YC, Zhou JJ, Chen YZ, Xie TA, et al. The clinical diagnostic value of Xpert MTB/RIF for the detection of Mycobacterium tuberculosis in gastric aspirates. Biosci Rep. 2020 Jun;40(6). Available from: https://pubmed.ncbi.nlm.nih.gov/32543657/
- Andronikou S, Grier D, Minhas K. Reliability of chest radiograph interpretation for pulmonary tuberculosis in the screening of childhood TB contacts and migrant children in the UK. Clin Radiol. 2021 Feb;76(2):122–8. Available from: https://pubmed.ncbi.nlm.nih.gov/33010931/
- García-Basteiro AL, López-Varela E, Augusto OJ, Gondo K, Muñoz J, Sacarlal J, et al. Radiological findings in young children investigated for tuberculosis in Mozambique. PLoS One. 2015;10(5):e0127323. Available from: https://pubmed.ncbi.nlm.nih.gov/26020541/
- Lozano-Acosta MM, Rubiano-Arenas MA, Cadavid LM, Vélez-Parra G, Molinares B, Marín-Pineda DM, et al. Reproducibility of a protocol for standardized reading of chest X-rays of children household contacts of patients with tuberculosis. BMC Pediatr. 2022 May;22(1):307. Available from: https://pubmed.ncbi.nlm.nih.gov/35610599/
- Dejene T, Hailu G, Kahsay A, Wasihun A. Pulmonary tuberculosis and rifampicin-resistant Mycobacterium tuberculosis in children and adolescents using GeneXpert MTB/RIF assay in Tigray, Northern Ethiopia. Infect Drug Resist [Internet]. 2023 Oct;16:6757–65. Available from: https://www.dovepress.com/pulmonary-tuberculosis-and-rifampicin-resistant-mycobacterium-tubercul-peer-reviewed-fulltext-article-IDR
- Zanozin AS, Berezovsky YS, Kogan EA, Kochetkova SE. Primary tuberculosis with miliary hematogenous generalization in old age. Clin Med (Russian Journal) [Internet]. 2023 Mar 27;101(1):63–7. Available from: https://www.clinmedjournal.com/jour/article/view/501
- Sorsa A. The diagnostic performance of chest X-ray and erythrocyte sedimentation rate in comparison with GeneXpert® for tuberculosis case notification among patients living with HIV in a resource-limited setting: A cross-sectional study. Risk Manag Healthc Policy [Internet]. 2020 Sep;13:1639–46. Available from: https://www.dovepress.com/the-diagnostic-performance-of-chest-x-ray-and-erythrocyte-sedimentatio-peer-reviewed-article-RMHP
- Wekesa C, Kirenga BJ, Joloba ML, Bwanga F, Katamba A, Kamya MR. Chest X-ray vs. Xpert® MTB/RIF assay for the diagnosis of sputum smear-negative tuberculosis in Uganda. Int J Tuberc Lung Dis [Internet]. 2014 Feb;18(2):216–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24429316
- Los Angeles County Department of Public Health. Tuberculosis (TB) and COVID-19 [Internet]. 2023. Available from: http://publichealth.lacounty.gov/docs/TB.FAQ.3.1.13.pdf
- Mousquer GT, Peres A, Fiegenbaum M. Pathology of TB/COVID-19 co-infection: The phantom menace. Tuberculosis [Internet]. 2021 Jan;126:102020. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1472979220301876
- Burrill J, Williams CJ, Bain G, Conder G, Hine AL, Misra RR. Tuberculosis: A radiologic review. RadioGraphics [Internet]. 2007 Sep;27(5):1255–73. Available from: http://pubs.rsna.org/doi/10.1148/rg.275065176
Medical Journal of Zambia, Vol 52, 2
The Medical Journal of Zambia, ISSN 0047-651X, is published by the Zambia Medical Association.
© This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.