Prevalence And Clinical Features of RSV Bronchiolitis In Hospitalised Children Under Two Years at the UTH Children’s Hospital, Lusaka, Zambia
Yolanta Banji Lilanda
University Teaching Hospital – Children’s Hospital, Lusaka Zambia
Veronica Mulenga
University Teaching Hospital – Children’s Hospital, Lusaka Zambia
Paul Simusika
Ministry of Health, pathology and Microbiology laboratory, University Teaching Hospital Lusaka, Zambia
Patrick Kaonga
University of Zambia, SACORE, Ridgeway campus, Lusaka, Zambia
Hitoshi Oshitani
Tohoku University, Graduate school, Department of Virology, Sendai, Japan
Evans Mpabalwani
University of Zambia, School of Medicine, Department of Paediatrics and child health, Lusaka, Zambia
DOI: https://doi.org/10.55320/mjz.52.2.637
Keywords:RSV Bronchiolitis, Prevalence, clinical features, risk factors, severity
ABSTRACT
Background
Bronchiolitis is a major cause of hospitalization in children under the age of two, and it occurs primarily in the winter, though it can occur at any time of year. The bulk of the causes are viral, with Respiratory Syncytial Virus (RSV) being the most common. RSV has been linked to a low fatality rate but a high morbidity rate, which includes severe symptoms, and a lengthy hospital stay. The goal of the study was to find out about bronchiolitis trends, the prevalence of RSV bronchiolitis, its clinical features, and the impact on severity and hospital stay.
Methods
A cross-sectional study was done on children less than two years admitted with clinical bronchiolitis to UTHs-CH, Lusaka Zambia. Nasopharyngeal (NP) swabs were collected and samples analysed by rRT-PCR for RSV. Risk factors and severity of bronchiolitis were determined on admission while length of stay, HIV status and secondary outcome (died, still in hospital, discharged) were determined on day 7 of hospital admission.
Results
We enrolled 181 Children with median age five months. Two-thirds were male and more than 90% had a mother alive. The prevalence of RSV was 25.4% (95% CI; 19.5-32.2). The highest number of patients were in April while the lowest numbers were observed in July in RSV negative and in June for RSV positive. More than two thirds had severe symptoms and congenital heart disease was significantly found to be risk factor for severe symptoms (P value 0.010). No independent clinical features were associated with RSV and no difference was found in severity between RSV positive and RSV negative bronchiolitis. The median length of hospital stay was five days and six days for RSV negative and RSV positive respectively and case fatality rate of RSV bronchiolitis was 2.2%.
Conclusion
The 25.4% laboratory confirmed detection rate for RSV during the study period may be low. Because the samples were taken during the COVID-19 pandemic, interventional measures to combat COVID-19 may have helped to reduce the spread of RSV. Because clinical features of RSV are like those of other respiratory viruses, a clinical diagnosis may be impossible, necessitating the use of PCR to confirm the diagnosis. Children with CHD are at significant risk of RSV bronchiolitis and would therefore benefit from preventive measures.
INTRODUCTION
Bronchiolitis is a viral acute lower respiratory tract infection (ALRI) occurring in early childhood with coughing, wheeze and poor feeding as the major symptoms.[1][2] It occurs mainly in winter and is considered one of the earliest and most common causes of hospitalization among young children during their first 2 years of life.[3] The clinical presentation of bronchiolitis includes rhinitis, cough, tachypnoea, use of accessory respiratory muscles, hypoxia, and variable wheezing and crepitations on auscultation.[4] Causative agents are viral, with respiratory syncytial virus (RSV) as the major cause.[5][6] With the utilization of PCR, the detection of specific viral nucleic acids has facilitated a better understanding of the viral aetiology of the infection. Assessment of the severity of bronchiolitis through a combination of clinical symptoms and physical signs remains a standard measure in daily practice.
The goals of the study were to determine the trends of admission for bronchiolitis, the prevalence of RSV bronchiolitis, its clinical features, associations with disease severity, and to compare the length of hospital stay between RSV-positive and RSV-negative bronchiolitis.
METHODS
The study was conducted in the admission ward of the University Teaching Hospital – Children’s Hospital, a tertiary hospital in Lusaka, with a bed capacity of 350. The prevalence formula was used to calculate the sample size, and a sample size of 205 was calculated.[7] Using consecutive sampling, we enrolled patients less than 24 months old who were admitted for less than 7 days with a diagnosis of bronchiolitis after consent from the caregiver. All children above 24 months or admitted with conditions other than bronchiolitis were excluded. To minimize selection bias, the nurses were fully trained on the enrolment process, there was a clearly defined criterion for the patients to be enrolled, and an effort was made to enrol all who qualified once consent was given.
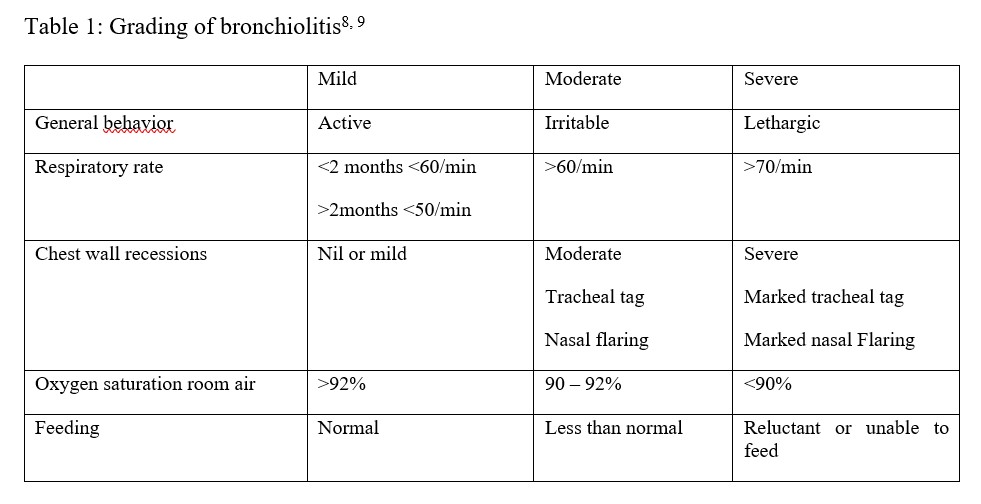
The clinical information was collected using a questionnaire, and after examination, the severity of illness was graded using the grading in Table 1. Grading was scored according to the highest parameter the patient exhibited. A nasopharyngeal swab was then collected and inserted into Viral Transport Medium, and specimens were sent to the virology laboratory for analysis using real-time reverse transcriptase PCR (rRT-PCR) for RSV. The RSV result was indicated as either positive or negative. A follow-up review on day 7 after admission was conducted, and it was documented whether the patient was still admitted, discharged, or had died. Other comorbidities such as HIV status, congenital heart disease, and chronic lung disease were also documented during follow-up. Data was entered into Excel and exported into Stata version 15 for analysis. All continuous variables such as age and the number of family members were tested for normality using the Shapiro-Wilk Test and found to be not normally distributed; therefore, for descriptive statistics, the median and interquartile range were reported. Frequency and percentages were reported for categorical variables such as sex. To determine the association between the outcome variable (RSV-positive) and any of the categorical variables when any of the cell frequencies was five or above, such as sex or the presence of a smoker, the chi-square test was used. However, in situations where the assumptions of the chi-square test were violated, with a frequency in any of the cells less than 5 (e.g., HIV status and atopy), the Fisher Exact test was used. To compare a continuous variable between RSV-positive and RSV-negative groups, the Mann-Whitney test was used. To determine the demographic and clinical factors associated with RSV bronchiolitis, multivariable logistic regression was used.
Ethical Considerations
Ethical approval to conduct the study was granted by UNZABREC and the National Health Research Authority. Further, permission for the study was obtained from the UTHs-CH Management. Children whose parents or guardians did not agree to participate in the study were excluded. Informed consent was obtained from guardians and parents.
RESULTS
The study was conducted between April and September 2020, and a total of 181 of the 205 patients were enrolled. The overall median age was four months. Table 2 shows the demographic characteristics and associations with RSV of the participants. The prevalence of RSV bronchiolitis was found to be 25.41% (95% CI 19.5–32.2).
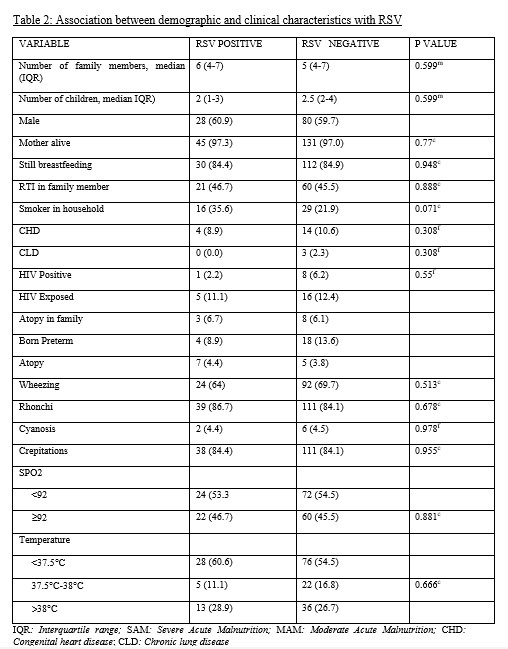
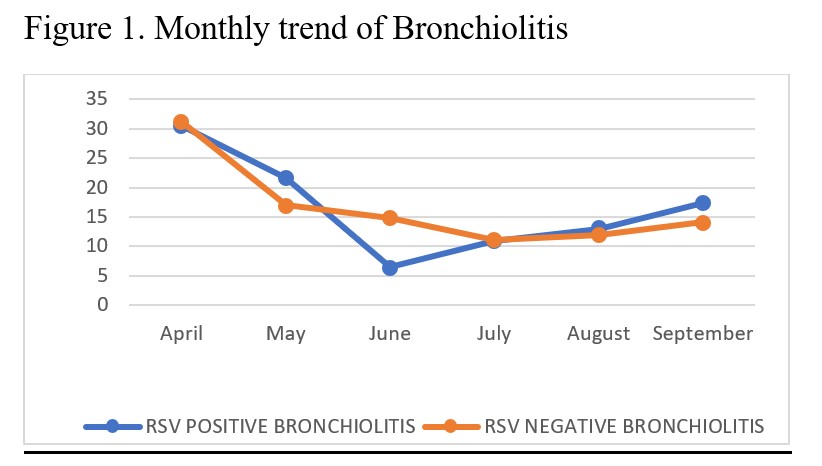
There was a downward trend of hospital admissions in both RSV-positive and RSV-negative groups from April to June, with a slight rise in August and September (Figure 1). There was no statistically significant difference between RSV-positive and RSV-negative groups based on clinical characteristics. In both groups, almost two-thirds of patients had severe symptoms (58.7% RSV-positive and 62.9% RSV-negative). Patients with Congenital Heart Disease (CHD) were found to be at significant risk of severe symptoms (94.4%) (P-value 0.010). Severe symptoms were also seen in patients with Chronic Lung Disease (CLD) (100%), Severe Acute Malnutrition (SAM), preterm babies (69%), those living in the same household as a smoker (60.9%), HIV-positive children (about two-thirds), and HIV-exposed children (48%). Patients with RSV bronchiolitis spent more time in the hospital (6 days, median IQR 4–11) than those with RSV-negative bronchiolitis (5 days, median IQR 4–9). RSV bronchiolitis had a case fatality rate of 2.2% (1/46).
DISCUSSION
This study recorded the highest number of bronchiolitis cases in April and the lowest in winter months, which is not usually the case in Zambia.[10] The study was conducted during the COVID-19 pandemic in Zambia. Measures such as handwashing, sanitizing, wearing face masks, and reducing overcrowding were implemented to curb the spread of COVID-19. These measures not only reduced the spread of COVID-19 but also of other viruses, hence the low turnover of patients.[11][12][13][14][15] A surge of bronchiolitis was noticed after the easing of measures and the reopening of schools in September 2020. This also showed that the spread of RSV can be reduced by hygiene measures such as handwashing, sanitizing, covering the mouth when sneezing, and reducing crowds.[14][15][16] The prevalence of RSV bronchiolitis was 25.4% (95% CI 19.5–32.2). This is slightly lower than the findings of the PERCH trial, which found the prevalence of RSV associated with wheeze to be 31.1%,[17] but higher than 13.5% in 1998 and 15% in 2015.[10][18]
Tissue culture was used in a study conducted at UTH in 1998, whereas PCR was used in this study. Because PCR has been proven to be more sensitive than tissue culture, the rise in prevalence can be explained.[19][20][21] Other studies conducted in Pakistan, Kenya, and Mozambique found the prevalence of RSV to be 19%, 15%, and 10.6%, respectively.[22][23] It was difficult to differentiate RSV bronchiolitis based on clinical features. Severity was comparable in both RSV-positive and RSV-negative groups. Other studies, however, have found RSV to be associated with wheezing and more severe disease.[24][25][26] CHD and CLD patients had severe symptoms. This is consistent with other studies that have demonstrated that patients with bronchopulmonary disease are predisposed to severe forms of bronchiolitis. This is because they have limited ability to increase their cardiac output in response to respiratory tract infection.[27][28] The hospital stay was observed to be 5 days for RSV-negative bronchiolitis and 6 days for RSV-positive bronchiolitis. There was no discernible difference between the two groups of patients. RSV bronchiolitis, on the other hand, has been linked to a longer hospital stay than bronchiolitis due to other viral causes.[29][30] The fatality rate in this study was 2.2%. Despite the low fatality rate, the typical length of stay of 6 days (median IQR 4–11) poses a significant challenge for hospital services, necessitating significant human resources, medicines, and oxygen supply to reduce mortality.[31][32] Policymakers should note the season in which RSV is expected to be on the rise. This will enhance proper planning for commodities to use within the hospital and human resource allocation. It will also give clinicians a high index of suspicion of RSV in children with bronchiolitis.
CONCLUSION
During COVID-19, 25.4% of UTHs-CH patients had RSV bronchiolitis. Bronchiolitis cases decreased from April to June. Based on clinical signs and severity, it was difficult to distinguish RSV-positive from RSV-negative bronchiolitis, and the average length of hospital stay was six days for RSV-positive bronchiolitis. CHD is significantly associated with severe RSV bronchiolitis. Public health interventions such as handwashing and hand sanitizing, masking, social distancing, and avoiding crowds should be emphasized to reduce the spread of respiratory viruses such as RSV. Clinicians should have a high index of suspicion of RSV in children with bronchiolitis and comorbidities such as CHD, SAM, prematurity, and HIV. It would also be of great importance to begin considering the introduction of an RSV vaccination for children under the age of two years old in Zambia, given the high prevalence and long hospital stay associated with RSV.
ACKNOWLEDGEMENTS
The authors greatly acknowledge the patients and the healthcare workers who made this work possible, as well as the University Teaching Hospital – Children’s Hospital management.
Abbreviations
ART: Antiretroviral Therapy, ALRI: Acute Lower Respiratory Tract Infection, CH: Children’s Hospital, DNA-PCR: Deoxyribonucleic Acid Polymerase Chain Reaction, HIV: Human Immunodeficiency Virus, MAM: Moderate Acute Malnutrition, NP: Nasopharyngeal, OP: Oropharyngeal, PCR: Polymerase Chain Reaction, rRT-PCR: Reverse Transcriptase – Polymerase Chain Reaction, RR: Respiratory Rate, RSV: Respiratory Syncytial Virus, SAM: Severe Acute Malnutrition, SPO2: Oxygen Saturations, UTHs-CH: University Teaching Hospitals – Children’s Hospital
Competing Interests
The authors declare that they have no competing interests.
Authors Contributions
Yolanta Banji Lilanda conceived the study together with Evans Mpabalwani and Veronica Mulenga, while Paul Simusika and Yolanta Banji Lilanda conducted the laboratory investigations. Patrick Kaonga performed the data analysis.
Disclaimer
The views expressed are those of the authors.
REFERENCES
- Holubkov R, Ph D, Reeves SD, Ruddy RM, Malik B, Nelson KA, et al. A multicentre Randomised, Controlled trial of Dexamethasone for Bronchiolitis. N Engl J Med. 2007 Jul 26;357(4):331-9. doi: 10.1056/NEJMoa071255. Erratum in: N Engl J Med. 2008 Oct 30;359(18):1972. Majahan, Prashant [corrected to Mahajan, Prashant]. PMID: 17652648.
- Schuh S, Babl FE, Dalziel SR, Freedman SB, Macias CG, Stephens D, et al. Practice variation in acute bronchiolitis: A paediatric emergency research networks study. Paediatrics. 2017;140(6).
- Shi T, McAllister DA, Brien KLO, Simoes EAF, Madhi SA, Gessner BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017 Sep 2;390(10098):946-958. doi: 10.1016/S0140-6736(17)30938-8. Epub 2017 Jul 7. PMID: 28689664; PMCID: PMC5592248.
- Wagner T, Disclosure A. Bronchiolitis Summary of American Academy of Paediatrics Clinical Practice Guidelines for Diagnosis and Management of Bronchiolitis. 2018;30(10).
- Moodley T, Masekela R, Kitchin O, Risenga S, Green RJ. Acute viral bronchiolitis: aetiology and treatment implications in a population that may be HIV co-infected. South African J Epidemiol Infect. 2010;25(2):6–8.
- Mustafa G. Bronchiolitis: the recent evidence. J Ayub Med Coll Abbottabad. 2014 Oct-Dec;26(4):602-10. PMID: 25672197.
- Arya R, Antonisamy B, Kumar S. Sample size estimation in prevalence studies. Indian J Pediatr. 2012;79(11):1482–8.
- Byrnes C et al. Wheeze and Chest Infection in infants under 1 year; Best Practice evidence-based guideline. Paediatric Society of New Zealand. 2005. pp 20-22.
- Øymar K, Skjerven HO, Mikalsen IB. Acute bronchiolitis in infants, a review. Scand J Trauma Resusc Emerg Med. 2014 Apr 3;22:23. doi: 10.1186/1757-7241-22-23. PMID: 24694087; PMCID: PMC4230018.
- Saijo M, Terunuma H, Mizuta K, Mpabalwani EM, Monze M, Oshitani H, Luo N, Suzuki H, Numazaki Y. Respiratory syncytial virus infection in children with acute respiratory infections in Zambia. Epidemiol Infect. 1998 Oct;121(2):397-400. doi: 10.1017/s0950268898001228. PMID: 9825792; PMCID: PMC2809538.
- Baker RE, Park SW, Yang W, Vecchi GA, Jessica C, Grenfell BT. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci U S A. 2020;117(48):30547–53.
- Trenholme A, Webb R, Lawrence S, Arrol S, Taylor S, Ameratunga S, et al. COVID-19 and infant hospitalizations for seasonal respiratory virus infections, New Zealand, 2020. Emerg Infect Dis. 2021;27(2):641–3.
- Oster Y, Michael-Gayego A, Rivkin M, Levinson L, Wolf DG, Nir-Paz R. Decreased prevalence rate of respiratory pathogens in hospitalized patients during the COVID-19 pandemic: possible role for public health containment measures? Clin Microbiol Infect. 2020 Dec 31;27(5):811–2. doi: 10.1016/j.cmi.2020.12.007. Epub ahead of print. PMID: 33352303; PMCID: PMC7833997.
- Britton PN, Hu N, Saravanos G, Shrapnel J, Davis J, Snelling T, et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc Heal. 2020;4(11):e42–3.
- Chou R, Dana T, Jungbauer R, Weeks C, McDonagh MS. Masks for Prevention of Respiratory Virus Infections, Including SARS-CoV-2, in Health Care and Community Settings: A Living Rapid Review. Ann Intern Med. 2020;173(7):542–55.
- Lieberthal AS, Bauchner H, Hall CB, Johnson DW, Kotagal U, Light MJ, et al. Diagnosis and management of bronchiolitis. Paediatrics. 2006;118:1774–93.
- Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019 Aug 31;394(10200):757-779. doi: 10.1016/S0140-6736(19)30721-4. Epub 2019 Jun 27. Erratum in: Lancet. 2019 Aug 31;394(10200):736. doi: 10.1016/S0140-6736(19)32010-0. PMID: 31257127; PMCID: PMC6727070.
- Simusika P, Bateman AC, Theo A, Kwenda G, Mfula C, Chentulo E, et al. Identification of viral and bacterial pathogens from hospitalized children with severe acute respiratory illness in Lusaka, Zambia, 2011-2012: A cross-sectional study. BMC Infect Dis. 2015;15(1):1–10.
- Perkins SM, Webb DL, Torrance SA, El Saleeby C, Harrison LM, Aitken JA, et al. Comparison of a real-time reverse transcriptase PCR assay and a culture technique for quantitative assessment of viral load in children naturally infected with respiratory syncytial virus. J Clin Microbiol. 2005;43(5):2356–62.
- Falsey AR, Formica MA, Treanor JJ, Walsh EE. Comparison of quantitative reverse transcription-PCR to viral culture for assessment of respiratory syncytial virus shedding. J Clin Microbiol. 2003;41(9):4160–5.
- Zahran WA, Makled AF, Salama AA. Comparison of Reverse Transcription - PCR and Viral Culture for Detection of Respiratory Syncytial Virus in Young Children: Relation to Epidemiological and Clinical Findings. Egypt J Med Microbiol. 2017;26(2):27–36.
- Ali A, Yousafzai MT, Waris R, Jafri F, Aziz F, Abbasi IN, Zaidi A. RSV associated hospitalizations in children in Karachi, Pakistan: Implications for vaccine prevention strategies. J Med Virol. 2017 Jul;89(7):1151-1157. doi: 10.1002/jmv.24768. Epub 2017 Feb 24. PMID: 28092107; PMCID: PMC5805860.
- Nokes DJ, Ngama M, Bett A, Abwao J, Munywoki P, English M, et al. Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin Infect Dis. 2009;49(9):1341–9.
- Ding Q, Xu L, Zhu Y, Xu B, Chen X, Duan Y, et al. Comparison of clinical features of acute lower respiratory tract infections in infants with RSV/HRV infection, and incidences of subsequent wheezing or asthma in childhood. BMC Infect Dis. 2020;20(1):1–9.
- Papenburg J, Boivin G. The distinguishing features of human metapneumovirus and respiratory syncytial virus. Rev Med Virol. 2010;20(4):245–60.
- Manoha C, Espinosa S, Aho SL, Huet F, Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J Clin Virol. 2007;38(3):221–6.
- Meissner HC. More on Viral Bronchiolitis in Children. N Engl J Med. 2016;375(12):1199–200.
- Karampatsas K, Kong J, Cohen J. Bronchiolitis: An update on management and prophylaxis. Br J Hosp Med. 2019;80(5):278–84.
- Hervás D, Reina J, Yañez A, Del Valle JM, Figuerola J, Hervás JA. Epidemiology of hospitalization for acute bronchiolitis in children: Differences between RSV and non-RSV bronchiolitis. Eur J Clin Microbiol Infect Dis. 2012;31(8):1975–81.
- Jartti T, Aakula M, Mansbach JM, Piedra PA, Bergroth E, Koponen P, et al. Hospital length-of-stay is associated with rhinovirus aetiology of bronchiolitis. Pediatr Infect Dis J. 2014;33(8):829–34.
- Pelletier AJ, Mansbach JM, Camargo CA. Direct medical costs of bronchiolitis hospitalizations in the United States. Paediatrics. 2006;118(6):2418–23.
- Unger S, Cunningham S. Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008 Mar;121(3):470-5. doi: 10.1542/peds.2007-1135. PMID: 18310194.
Medical Journal of Zambia, Vol 52, 2
The Medical Journal of Zambia, ISSN 0047-651X, is published by the Zambia Medical Association.
© This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.