Determinants of Oral Squamous Cell Carcinoma among Suspected Cases at The Maxillo-Facial Unit of the Adult University Teaching Hospitals in Lusaka, Zambia
Dr. Jessy Mutale Nkonde
The University of Zambia
Dr. Christopher Kapeshi
The University Teaching Hospitals (Adult)
Joseph Lupenga
The University of Zambia
DOI: https://doi.org/10.55320/mjz.52.1.615
Keywords: oral squamous cell carcinoma, determinants
ABSTRACT
Oral Squamous Cell Carcinoma (OSCC) accounts for nearly 90% of all oral cancers. In resource-constrained settings, such as Zambia, most contributing factors to oral squamous cell carcinoma are unknown. Therefore, this study aimed to identify determinants of Oral Squamous Cell Carcinoma in patients presenting to the Maxillo-Facial Unit at the Adult University Teaching Hospital in Lusaka, Zambia.
MethodsA cross-sectional study was conducted from June to December 2020. A systematic random sampling was used to identify 249 eligible participants from whom data was collected using a validated structured questionnaire. Determinants of Oral Squamous Cell Carcinoma were identified in a multivariable logistic regression analysis.
ResultsThe prevalence of Oral Squamous Cell Carcinoma was 42.1% (95% CI: 36% - 48%). Grade II (moderately differentiated, n=68, 64%) histopathology subtype was the most prevalent. Determinants of Oral Squamous Cell Carcinoma were increasing age (AOR: 1.19, 95% CI: 1.08–1.32), being male (AOR: 2.04, 95% CI: 1.03–4.04), tobacco use (AOR: 3.49, 95% CI: 1.34–9.05), low BMI (AOR: 3.80, 95% CI: 1.52–9.52), and having the disease for more than six months (AOR: 2.21, 95% CI:1.06–4.60). Higher BMI (AOR: 0.48, 95% CI: 0.23-0.99) and poor oral hygiene (AOR: 0.35, 95% CI: 0.18-0.69) were associated with reduced risk.
ConclusionAt the Maxillo-Facial Unit, 42.1% of suspected patients had Oral Squamous Cell Carcinoma. Tobacco prevention should be prioritised, as should promoting early health-seeking attitudes and health education, especially among males.
INTRODUCTION
Oral Cancer is a global disease and one of the main causes of death, with over 300,000 new cases and approximately 100,000 deaths recorded in 2018 [1],[2]. Oral Cancer incidence has increased globally in different regions, ranging from 0.4 to 3.3% annually [3]. Oral Squamous Cell Carcinoma (OSCC) is the most common form of cancer in the oral cavity, accounting for more than 90% of all oral malignancies, with a strong predilection for males aged forty and above [4],[5].
OSCC was traditionally an age and sex defined pathology in males aged 40 years and above, but recent research has revealed a shift in age and sex at presentation in those 40 years and below. Early exposure to risky behaviours such as smoking (especially shisha smoking in females), alcohol use, severe oral infection, and genetic predisposition, has been attributed to this change [6],[7]. Poor health-seeking behaviour, poor Oral Cancer knowledge, and social economic constraints, such as lack of health insurance or unemployment, are known social determinants of OSCC.
The findings from a systematic study conducted by Faggons and colleagues revealed that despite the widespread distribution of OSCC cases, the precise causes of OSCC are unknown, and determinants vary and are unidentified in various settings [8]. The consequences of not knowing or identifying OSCC determinants affect individualised patient care, which impacts policy formulation. Early identification of OSCC determinants should precede awareness campaigns, especially regarding Oral Cancer prevention and the self-examination diagnosis of OSCC, like how other cancers, such as breast and prostate cancer, are encouraged [9]. This approach promotes early health-seeking attitudes and health education, especially among males and those exposed to modifiable cancer risky habits, such as tobacco and alcohol use.
With Zambia experiencing a significant burden of cancer-related conditions, a study by Zyaambo et al. (2013) reported that, among 12,891 cancer cases that were reviewed in the Zambia National Cancer Registry (1990–2009), Head and Neck Cancers accounted for 10% (533), with a notable male dominance at 8% (324) [10]. The reported prevalence of Head and Neck Cancers may be associated with poor documentation (in record entries) and incomplete data, especially for OSCC.
This highlights the need to strengthen Cancer research and reporting in regional and National Cancer Registries to improve patient care and management, as suggested by Kayamba et al. (2021) in their systematic review on cancers in sub-Saharan Africa with Zambia as an example. While Zambia has made significant advancements in cancer research and documentation, particularly for Prostate, Cervical, and Breast cancers, as well as Kaposi’s sarcoma and Oesophageal Squamous Cell Carcinoma [11]. As far as literature was reviewed, data on Oral Cancers remain outdated, underreported, and incomplete, likely underestimating the true burden of the disease in Zambia.
This study aimed to determine the prevalence, determinants, and common histological subtypes of OSCC among patients at the Maxillo-Facial Unit of the Adult University Teaching Hospitals in Lusaka. It provides baseline data to improve early detection and management of OSCC and lays the groundwork for future longitudinal studies, such as cohort studies, which can provide more evidence to infer association of the identified determinants.
METHODS
Study Design and Setting
A cross-sectional study was conducted at the Adult University Teaching Hospitals in Lusaka at the Maxillo-Facial Unit. The University Teaching Hospital is a referral hospital and tertiary learning institution. Its proximity to the National Cancer Disease Hospital adds to its appeal in multidisciplinary management. The five sections of the University Teaching Hospitals in Lusaka, Zambia are Adult Hospital, Paediatric Hospital, Eye Hospital, Women and New-Born Hospital, and Cancer Disease Hospital. The Maxillo-Facial Unit is a unit under the University Teaching Hospitals Adult Hospital. This Unit is staffed with trained and specialised personnel capable of managing a wide range of complex and simple oral-related conditions in both adults and children, as well as malignant and benign lesions.
Study Population and Sample Size
This study included all patients who were referred to the Maxillo-Facial Unit from various health centres, as well as those who were presented to the Maxillo-Facial Unit with suspected OSCC. Those who did not provide written informed consent or whose biopsy results were missing were excluded from the study. The estimated sample size was 266 participants which was estimated using a Cochrane single proportion formula.
Where: n= (𝑍². 𝑃 (1−𝑃)) /𝑒²
n= sample size
Z = confidence coefficient (1-α) at 1.95
P= proportion 20.8% (0.208) adopted from a study done in Zimbabwe[12].
e= error of margin (0.05)
During the six-month data collection period, a sample size of 266 was expected. Systematic random sampling was used to sample participants, and the sampling interval was determined to be K=2 (N/n; 480/266=2). The first participant was randomly chosen from the sampling frame, and consecutive participants were selected at a fixed interval determined (k=2) until the stipulated sample was attained. By selecting participants at a given interval, selection bias was minimized.
The main obstacle to not reaching the expected sample size was the emergence of the Covid 19 pandemic and its limitation. The limitation arose from the public health recommendation to restrict treatments and patient inflow to elective cases only, leading to a low patient turnout. However, in awarding the data internal validity, from June to December 2020, 249 patients were sampled.
Data Collection
A previously validated structured questionnaire was used to collect data from participants on socio-demographics, patient-related characteristics, and histopathology subtypes. The presence or absence of Oral Squamous Cell Carcinoma (OSCC) was the outcome variable based on histopathology confirmation. Broaders grading systems were employed in the present study[13]. Explanatory variables were age, gender, educational level, marital status, employment status, residential population density, source of medical information, Oral Cancer knowledge, Oral Cancer screening, family history of cancer, oral hygiene, oral infection, HIV status, and comorbidity.
Age was collected as a continuous variable in years and months and analysed as a continuous variable to show the impact of a unit increase in age. Age was further divided into three groups (1–5 years, 16–39 years, and 40 years and above) to demonstrate discrepancies in the trends identified at disease presentation. The subgroup groups were selected based on a review of the literature on Oral Squamous Cell Carcinoma presentation[14]. The residential population density was divided into three categories (low, medium, and high) based on data from the central statistics office Zambia demography and health survey (DHS)[15].
Oral hygiene was assessed in two categories using the decayed missing filled teeth (DMFT) index and oral hygiene simplified index (OHS-I). Good oral hygiene was defined as a DMFT index of 0-3 and an OHS-I of 0-1. Poor oral hygiene was defined as a DMFT index of 4 or greater and an OHS-I of 2 or greater. This classification was necessary to demonstrate the differences in the predisposition to Oral Cancer in these groups of patients[16].
Data Analysis
STATA 14 (Stata Corp., College Station, Texas, USA) was used for all analyses. Frequencies and percentages were used to represent estimates of categorical variables. Age was normally distributed, Shapiro-Wilk test was used to check the normality of the distribution of age (p=0.186); in this regard, the mean and standard deviation were reported. The presence of OSCC was coded as a binary outcome variable (yes or no).
Univariate and multivariable logistic regression analysis were used to identify the determinants of OSCC and control for potential confounders and biases using an investigator led approach. The main explanatory variables were age, sex, place of residence, oral hygiene, smoking, alcohol use, exposure to human immunodeficiency virus, body mass index, and lesion duration. The level of significance was set at 5% and the confidence level at 95%. Therefore, a p-value less than 0.05 was considered statistically significant. Crude and adjusted odd ratios at a 95 % confidence interval were reported. The best model was selected using predictive power measures, such as the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC). The model with the lowest AIC and BIC was selected as the final model.
Ethical Considerations
The University of Zambia Biomedical Research Ethics Committee granted ethical approval (UNZABREC reference number 1028-2020) and the National Health Research Authority of Zambia (NHRA). A unique identification code (or masking) was assigned to each medical record, with no names recorded. The data collected was stored on a two-factor authentication-protected computer with the principal investigator to ensure confidentiality. Additionally, informed consent was obtained from all eligible participants.
RESULTS
Demographic and Clinical Characteristics
The study included 249 patients with suspected OSCC. Over half of the patients were aged 40 years and above 133 (53.4%) with the mean age of 39.5 years±18.3. The composition of the sample was predominantly male 156 (62.7%). Most patients were unemployed 202 (81%), and half were from high-density areas 137(55.0%). Over a third of the patients were alcohol users 94 (37.8%) and 57 (22.9%) used tobacco. Most patients 221 (88.8%) had poor knowledge of Oral Cancer, (Table 1).
Oral Squamous Cell Carcinoma Rate by Demographic and Clinical Characteristics
The prevalence of OSCC in suspected cases was 42.1% (105; 95% CI: 0.36-0.48). The prevalence of OSCC was higher in individuals who were 40 years and above 72 (54.1%), males 71 (67.6%), married 78 (50.1%), use alcohol 53 (56.4%), and use tobacco 42 (73.7%). Additionally, the rate of OSCC was higher in patients with low BMI 34 (69.4%), HIV positive 67 (37.6%), good oral hygiene 65 (49.2%), having comorbidities 86 (39.3%), hypertension 85 (39.0%), lesion for more than six months 83 (48.8%), high-risk precancerous lesions 94 (74.1%), and lesions on the floor of the mouth 20 (80.0%), lip 10 (66.7%), and maxillary buccal 54 (45.0%). However, no association of OSCC was observed with education level, housing density, employment status, sources of medical information, Oral Cancer screening, family history of cancer, oral infections, or diabetes (p > 0.005). (Tables 2 and 3).
Determinants of OSCC
OSCC was significantly associated with age, sex, oral hygiene, tobacco use, Body Mass Index (BMI), and lesion duration (p<0.05). Adjusting for possible confounders in multivariable analysis, OSCC determinants were increasing age (AOR: 1.19, 95 % CI: 1.08–1.32), being male (AOR: 2.04, 95 % CI: 1.03–4.04), tobacco use (AOR: 3.49, 95 % CI: 1.34–9.05), having a low BMI (AOR: 3.80, 95 % CI: 1.52–9.52), and having the disease for more than six months (AOR: 2.21, 95% CI:1.06–4.60). Poor oral hygiene (AOR: 0.35, 95% CI: 0.18–0.69) and a higher BMI (AOR: 0.48, 95% CI: 0.23–0.99) were associated with reduced risk of OSCC (Table 4).
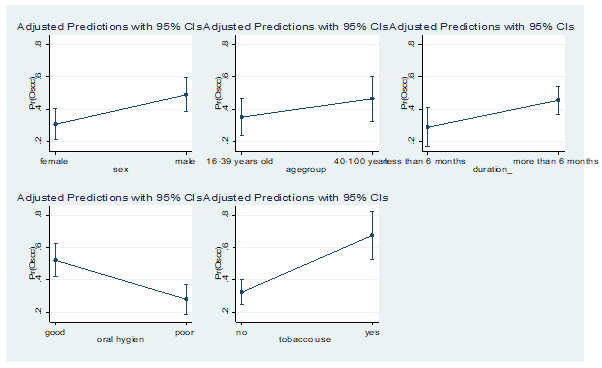
Margin Plots: Summary of Demographic and Clinical Factors
Figure 1: Predicted probabilities of sex, age, duration of the lesion, oral hygiene and tobacco use with OSCC.
The results in figure 1 show that the probability of developing OSCC is higher in males, patients (>40 years), patients with the disease more than six months and tobacco users. However, patients with poor oral hygiene are presented with a reduced probability of developing OSCC. The results further show that there is enough evidence to prove that the probabilities are different from zero (p<0.001).
Histopathology Subtypes of Confirmed OSCC Cases
Of the cases with confirmed OSCC, 68 (65%) cases were in category II (grade II), and keratinizing tumours were the most common subtype 86 (81.9%). (Table 5).
DISCUSSION
This study provides valuable insights into the prevalence, determinants, and histological subtypes of OSCC among patients with suspected OSCC. OSCC was confirmed in 42.1% of suspected OSCC cases, with the floor of the mouth being the most affected areas. The most common histological subtype was keratinising tumours, predominantly moderately differentiated (grade II). Key OSCC determinants included increasing age, male, tobacco use, low BMI, and disease duration of more than six months.
In this study, 42.1% of suspected cases were confirmed as OSCC, while the rest were diagnosed with other conditions, including oropharyngeal carcinoma, adenoid cystic carcinoma, Kaposi's sarcoma, osteomyelitis, and severe periodontal infection. However, although the prevalence of OSCC was lower than expected (based on anecdotal evidence), multivariable analysis indicated that patients who delay seeking health care, specifically those who report symptoms for six months or more before consulting a healthcare provider, were significantly at a higher risk of being diagnosed with OSCC, which was in concordance with the anecdotal predication of late presentation and advanced disease at presentation among suspected patients at the Maxillo-Facial Clinic of The University Teaching Hospitals.
OSCC remains the most prevalent form of oral and pharyngeal cancer globally[17]. Despite its widespread occurrence, OSCC is often detected at advanced stages, leading to a consistently low five-year survival rate, hovering below 50% over the past three decades[18],[19]. Identifying predictors of OSCC, whether through individual vigilance or professional screening initiatives, is essential for improving patient outcomes and diagnostic delays.
In sub-Saharan Africa, the prevalence of OSCC is dependent on geographical variation of risk factors distribution[8]. However, the complexity of its risk factors has resulted in widely varying OSCC prevalence rates across regions in sub-Saharan Africa, where rates range from 10.8% to 43.7%[20],[21]. These disparities highlight the urgent need for region-specific prevention and screening interventions tailored to local challenges.
Key actions include promoting timely patient presentation to health facilities, ensuring access to oral healthcare providers (Dentists), training healthcare workers in early detection of oral premalignant lesions, and equipping dental facilities with modern technologies for improved diagnosis and screening. Additionally, investing in Oral Cancer research and heightening preventative measures of modifiable Oral Cancer risk factors like tobacco and alcohol use is recommended.
In this study, 74.0% of presenting patients with suspected OSCC had high-risk cancer lesions for over 6 months, of which 42.1% were historically confirmed as OSCC. Similarly, a study in Iran found that delays due to patients were more substantial than those from healthcare professionals among Oral Cancer cases[22], while in Kenya, late presentations of Oral Cancers were linked to a restrictive referral system[23]. These findings align with the current study, which significantly found that both patient and professional factors significantly contributed to diagnostic delays in most OSCC cases after adjusting for biases and confounders in regression analysis.
There are several factors that influence late presentation, of which patient presentation remains the most highlighted, contributing to diagnostic setbacks in OSCC management. However, clinician delays in OSCC diagnosis are also another contributing factor to the poor disease-specific prognosis, which often results from misinterpreting early lesions as benign, impacting prognosis and treatment outcomes[24].
The present study further revealed that the prevalence of OSCC was significantly associated with patient-related factors such as being male, particularly in individuals over 40 years of age. This demographic is often characterized by delayed health-seeking behaviours and greater exposure to risk factors such as tobacco use[9]. The findings support established research linking OSCC to age-related risk, with incidence increasing due to cumulative exposure to carcinogens and aging[19].
However, men over 40 are especially susceptible, not only because of prolonged exposure to habits like alcohol and tobacco use but also due to genetic predispositions that appear to elevate cancer risk post-40 years[25]. To improve early detection, Oral Cancer education and screening should be integrated into routine health services for high-risk groups (males > 40 years) and implemented in all health centres. Tobacco prevention and cessation programs must be strengthened and actively promoted, leveraging social and digital platforms and local engagements. Collaboration with cancer awareness campaigns, such as those for prostate cancer, can increase community awareness of Oral Cancer risk factors and support the early detection of precancerous oral lesions.
This study found that other factors inherent to the patient such as poor oral hygiene showed a protective effect, which contrasts with existing literature linking poor oral hygiene to carcinogenic microorganisms in the oral cavity[26]. This discrepancy suggests a need for further longitudinal research, perhaps through case-control studies, to examine possible measurement biases. Nonetheless, maintaining good oral hygiene remains essential for overall oral health and reducing disease risk.
Multivariable analysis of the present study further revealed that patient-related factors such as BMI was associated with an increased risk of developing OSCC. Underweight patients faced a higher risk compared to those with normal BMI, while being overweight appeared protective. Prior research also associated low BMI with poorer survival and surgical outcomes in advanced OSCC due to nutritional challenges[27],[28]. Conversely, another study found that while BMI is sometimes associated with cancer recurrence due to complicating comorbidities such as obesity, it was not identified as a prognostic indicator for patients with squamous cell carcinoma of the head and neck[29]. Our findings reinforce the connection between low BMI and increased OSCC risk, emphasizing how this affects treatment outcomes, as well as patients' nutritional and mental health.
In this study, tumour-related factors, such as tumour site, were associated with the development of OSCC. Identifying the primary site of the tumour is an important disease progression prognosticator of OSCC[30]. Similarly, a study by Majumdar et al. revealed that tumour site is a prognostic determinant factor[30]. The prognostic variation of OSCC was attributed to varying vascularization, lymphatic networks, and histology differentiation within the oral cavity.
Their update review showed that OSCC of the lip, hard palate, and maxillary gingiva rarely invade regional lymphoid with poor prognosis, while the tongue and the floor of the mouth, mandibular gingival metastases to regional lymphoid and are aggressive with a less favourable prognosis[31].
However, identifying the primary site is not an easy task clinically, especially in advanced disease presentation coupled with the poor documentation of patient history by many clinicians and the patients recall biases. This is particularly more complex in OSCC due to the field defect characteristic of OSCC, which enables dysplastic changes across multiple areas of the oral cavity, especially in individuals with habits like tobacco use[32].
In concordance, a study by Lin and colleagues in Taiwan argues the need to consider specific biomarkers to further explain the differences associated with prognosis in different subsites. It is clinically recommendable that biopsy samples are harvested from various regions and in depth of the tumour to improve the diagnosis sensitivity of OSCC[33].
The current study found that tumours of the floor of the mouth, lip, and maxillary were the most prevalent. Patients with tumours in these regions generally presented at advanced disease stages. This late presentation posed a challenge in identifying the primary sites of the tumour to predict clinical prognosis and metastasis. Conversely, another study revealed that there is no significant difference between the value of the classical Broders’ grading system and various prognostic outcome measures such as regional metastasis, local recurrence, and 5-year survival[34]; This simply implies that the primary tumour sites should be explored efficiently as a prognostic measure of disease-specific survival in OSCC.
The findings of this study affirm prior research which emphasized the need for improved early detection by exploring non-invasive, novel technological approaches. Which includes developing novel biomarkers specific to OSCC diagnosis and prognosis, taking a comprehensive history of presenting complaints, and integrating into routine practice risk factor assessments for oral cancers[35].
Other tumour-related factors identified in the present study were tumour types and grades, of which two-thirds of OSCC cases were moderately differentiated, with 81.9% classified as keratinising tumours. The current study did not include the degree of keratinising, a limitation inherent to the histopathology grading system used. However, findings from other researchers revealed that lower keratinisation levels have been associated with higher recurrence rates, highlighting the value of detailed histological examination in assessing OSCC prognosis[35],[36]. While implementing such detailed histopathological assessments remains a challenge in resource-constrained settings, this study suggests that exploring the degree of keratinisation could provide useful prognostic information, particularly in cases with late presentation.
This study highlights the need for individualized determinants of OSCC and multifaceted strategies to enhance early detection and outcomes. Prioritizing high-risk groups, such as men over 40 years, and incorporating factors like BMI, HIV status, and tumor characteristics into screening and educational sensitization programs could improve OSCC management and patient survival.
CONCLUSION
The present study, conducted at the University Teaching Hospital in Lusaka, Zambia, found that 42.1% of patients suspected of having OSCC were confirmed to have the disease, highlighting the urgent need for targeted prevention efforts, particularly in tobacco control, and for early detection among men. The study further highlights the need for improved Oral Cancer research and development in Zambia, the integration of Oral Cancer screening into routine health services or community outreach programs alongside other Non-Communicable Diseases (NCDs), and the contextualization of innovative technologies such as biomarkers into routine clinical diagnosis and investigation to improve the sensitivity and specificity of OSCC diagnosis.
Strength of the Study
This study had notable strengths, including eligibility assessments and biopsy procedures conducted by an experienced Dental Surgeon, with biopsy analysis carried out by knowledgeable pathologists following recommended criteria. These practices ensure the reliability of findings. Additionally, this study contributes to closing the gap in prevention and awareness, particularly for individuals engaging in modifiable risk behaviours, such as tobacco and alcohol use. These insights are valuable for developing targeted education and prevention strategies.
Limitations
The current study had several limitations. As a single facility-based study, it highlights the need for a multicentre approach to provide a more comprehensive understanding of OSCC and its determinants across diverse populations. Additionally, delays in patient presentation complicated the identification of primary tumour sites, affecting diagnostic accuracy. Collaborative efforts should focus more on increasing public awareness, education on early diagnosis and treatment, and training healthcare practitioners for timely interventions. Exploring novel techniques and technologies that are less invasive compared to biopsies, such as biomarkers, is encouraged for improving diagnostic accuracy.
What is Already Known on this Topic?
- Depending on environmental and social exposures, OSCC prevalence, histological subtypes, and determinants vary by region.
- Despite the well-defined tumour growth pathogenesis, patients with OSCC present late, and attempts at early detection by adjuvant diagnosis and screening have not lived up to the initial promises in most sub-Saharan Africa.
What This Study Adds
- This study provides baseline data that may inform guidelines for clinicians on the screening and management of OSCC and may help to identify the determinants of OSCC and raise awareness among those at risk of developing OSCC.
- The study will help to reduce the data gap on OSCC in Zambia, as well as promote prevention strategies on Oral Cancer especially among those exposed to modifiable risky cancer behaviours, such as tobacco and alcohol use and poor health seeking attitudes.
Competing Interest
The study had no competing interests or conflicts of interest, as this was a self-funded study.
Authors’ Contribution
J.M.N contributed substantially to conception and design, data acquisition, analysis, interpretation of data and drafting of the manuscript. C.K. contributed to the design, acquisition of data and revision of the manuscript. J.L. contributed substantially to the design, analysis, interpretation of data and drafting of the manuscript.
Acknowledgements
We would like to extend our sincere gratitude to all research participants and the staff at the Maxillofacial Clinic of the University Teaching Hospital Adult, Lusaka, Zambia, especially Sr. Maria. Special thanks to Dr. Songwe and Mr. Kennedy from the Histopathology Department for their support. A special thank you to Dr. Patrick Kaonga for his dedication and guidance, and to the entire faculty and colleagues at the University of Zambia, School of Public Health.
List of Tables
Main Tables
- Table 1: Baseline Characteristics of Patients.
- Table 2: Association of Baseline Characteristics With OSCC.
- Table 3: Clinical Characteristics of Patients with Suspected OSCC.
- Table 4: Determinants of OSCC.
Auxiliary Tables
- Table 5: Histopathology Subtypes and Tumour Sites of Confirmed OSCC Cases.
List of Figures
- Figure 1: Predicted probabilities of sex, age, duration of the lesion, oral hygiene and tobacco use with OSCC.
REFERENCES
- D’Cruz AK, Vaish R, Dhar H. Oral cancers: Current status. Oral Oncol. 2018 Dec 1;87:64–9. Available from: https://pubmed.ncbi.nlm.nih.gov/30527245/
- Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015 Jul 20;15(6):e6186.
- Ng JH, Iyer NG, Tan MH, Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck. 2017 Feb 1;39(2):297–304. Available from: https://pubmed.ncbi.nlm.nih.gov/27696557/
- Elaiwy O, El Ansari W, AlKhalil M, Ammar A. Epidemiology and pathology of oral squamous cell carcinoma in a multi-ethnic population: Retrospective study of 154 cases over 7 years in Qatar. Ann Med Surg (Lond). 2020 Dec 1;60:195–200. Available from: https://pubmed.ncbi.nlm.nih.gov/33163176/
- Rethman MP, Carpenter W, Cohen EEW, Epstein J, Evans CA, Flalfz CM, et al. Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. J Am Dent Assoc. 2010;141(5):509–20. Available from: https://pubmed.ncbi.nlm.nih.gov/20436098/
- Adoga AA, Kokong DD, Ma’an ND, Mugu JG, Mgbachi CJ, Dauda AM. The predictive factors of primary head and neck cancer stage at presentation and survival in a developing nation’s tertiary hospital. SAGE Open Med. 2018 Aug 1;6. Available from: https://pubmed.ncbi.nlm.nih.gov/30140440/
- Du M, Nair R, Jamieson L, Liu Z, Bi P. Incidence Trends of Lip, Oral Cavity, and Pharyngeal Cancers: Global Burden of Disease 1990-2017. J Dent Res. 2020 Feb 1;99(2):143–51. Available from: https://pubmed.ncbi.nlm.nih.gov/31874128/
- Faggons CE, Mabedi C, Shores CG, Gopal S. Review: Head and neck squamous cell carcinoma in sub-Saharan Africa. Malawi Med J. 2015 Sep 1;27(3):79–87. Available from: https://pubmed.ncbi.nlm.nih.gov/26715951/
- Nabukenya J, Hadlock TA, Arubaku W. Head and Neck Squamous Cell Carcinoma in Western Uganda: Disease of Uncertainty and Poor Prognosis. OTO Open. 2018;2(1). Available from: https://pubmed.ncbi.nlm.nih.gov/30480207/
- Zyaambo C, Nzala SH, Babaniyi O, Songolo P, Funkhouser E, Siziya S. Distribution of cancers in Zambia: Evidence from the Zambia national cancer registry (1990–2009). academia.edu. 2013;5(2):95–100. Available from: https://www.academia.edu/download/45865693/Distribution_of_cancers_in_Zambia_Eviden20160522-6150-10qzt41.pdf
- Kayamba V, Mutale W, Cassell ; Holly, Douglas Corbett Heimburger ; Shu XO. Systematic Review of Cancer Research Output From Africa, With Zambia as an Example. JCO Glob Oncol. 2021 Dec;7(7):GO.21.00079. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8459799/
- Chidzonga MM, Mahomva L. Squamous cell carcinoma of the oral cavity, maxillary antrum and lip in a Zimbabwean population: a descriptive epidemiological study. Oral Oncol. 2006;42(2):184–9. Available from: https://pubmed.ncbi.nlm.nih.gov/16256417/
- AC Broders. Carcinoma: Grading and practical application. ci.nii.ac.jp. 1926. Available from: https://ci.nii.ac.jp/naid/10018929621/
- Mneimneh WS, Xu B, Ghossein C, Alzumaili B, Sethi S, Ganly I, et al. Clinicopathologic Characteristics of Young Patients with Oral Squamous Cell Carcinoma. Head Neck Pathol. 2021 Dec 1;15(4):1099–108.
- Zambia Statistics Agency, Ministry of Health Zambia I. GOVERNMENT OF ZAMBIA Zambia Demographic and Health Survey 2018. 2019;1–581. Available from: www.DHSprogram.com.
- Katge F, Rusawat B, Shitoot A, Poojari M, Pammi T, Patil D. DMFT index assessment, plaque pH, and microbiological analysis in children with special health care needs, India. J Int Soc Prev Community Dent. 2015;5(5):383–8. Available from: https://pubmed.ncbi.nlm.nih.gov/26539390/
- Miranda-Filho A, Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020 Mar 1;102.
- Miller, B.A., Feuer, E.J. and Hankey, B.F., 1993.
- Asio J, Kamulegeya A, Banura C. Survival and associated factors among patients with oral squamous cell carcinoma (OSCC) in Mulago hospital, Kampala, Uganda. Cancers Head Neck. 2018 Dec;3(1).
- Adeyemi B, Kolude B, Akang E. A retrospective histopathological review of oral squamous cell carcinoma in a Nigerian teaching hospital. Afr J Med Med Sci. 2011.
- Jafari A, Najafi SH, Moradi F, Kharazifard MJ, Khami MR. Delay in the diagnosis and treatment of oral cancer. Journal of Dentistry. 2013 Sep;14(3):146. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3927673/
- Onyango JF, Macharia IM. Delays in diagnosis, referral and management of head and neck cancer presenting at Kenyatta National Hospital, Nairobi. East Afr Med J. 2006;83(4):85–91.
- Petruzzi MNMR, Cherubini K, Salum FG, de Figueiredo MAZ. Role of tumour-associated macrophages in oral squamous cells carcinoma progression: An update on current knowledge. Diagn Pathol. 2017 Apr 5;12(1).
- Polz-Gruszka D, Morshed K, Stec A, Polz-Dacewicz M. Prevalence of Human papillomavirus (HPV) and Epstein-Barr virus (EBV) in oral and oropharyngeal squamous cell carcinoma in south-eastern Poland. Infect Agent Cancer. 2015 Oct 12;10(1).
- Whitmore SE, Lamont RJ. Oral Bacteria and Cancer. PLoS Pathog. 2014;10(3).
- Chang WC, Yang CY, Lin CS, Lin CK, Chen YW. Pretreatment body mass index as a prognostic predictor in patients with oral squamous cell carcinoma. Clin Oral Investig. 2020 Aug 1;24(8):2781–8.
- Moon H, Roh JL, Lee SW, Kim SB, Choi SH, Nam SY, Kim SY. Prognostic value of nutritional and hematologic markers in head and neck squamous cell carcinoma treated by chemoradiotherapy. Radiotherapy and oncology. 2016 Feb 1;118(2):330-4.
- Li P, Sun L, Sun L. Influence of body mass index on survival and prognosis in squamous cell carcinoma of head and neck. Cancer Manag Res. 2020;12:3203–10.
- Braakhuis BJM, Brakenhoff RH, Leemans CR. Second Field Tumors: A New Opportunity for Cancer Prevention? Oncologist. 2005 Aug 1;10(7):493–500.
- Majumdar B, Patil S, Sarode SC, Sarode GS, Rao RS. Clinico-pathological prognosticators in oral squamous cell carcinoma. Translational Research in Oral Oncology. 2017 Jan 1;2:2057178X1773891.
- Mohan M, Jagannathan N. Oral field cancerization: an update on current concepts. Oncology reviews. 2014 Mar 3;8(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4419611/
- Lin NC, Hsien SI, Hsu JT, Chen MY. Impact on patients with oral squamous cell carcinoma in different anatomical subsites: a single-center study in Taiwan. Scientific Reports. 2021 Jul 29;11(1):15446. Available from: https://www.nature.com/articles/s41598-021-95007-5
- Weijers M, Snow GB, Dick Bezemer P, Van Der Waal I. Malignancy grading is no better than conventional histopathological grading in small squamous cell carcinoma of tongue and floor of mouth: Retrospective study in 128 patients. Journal of Oral Pathology and Medicine. 2009 Apr;38(4):343–7.
- Irimie AI, Braicu C, Cojocneanu-Petric R, Berindan-Neagoe I, Campian RS. Novel technologies for oral squamous carcinoma biomarkers in diagnostics and prognostics. Acta Odontol Scand. 2015 Apr 1;73(3):161–8.
- Wolfer S, Elstner S, Schultze-Mosgau S. Degree of Keratinization is an Independent Prognostic Factor in Oral Squamous Cell Carcinoma. Journal of Oral and Maxillofacial Surgery. 2018 Feb 1;76(2):444–54.
- Woolgar JA. Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2006 Mar;42(3):229–39. Available from: https://pubmed.ncbi.nlm.nih.gov/16150633/
Medical Journal of Zambia, Vol 52, 1
The Medical Journal of Zambia, ISSN 0047-651X, is published by the Zambia Medical Association.
© This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.