Determinants of Cervical Cancer Screening Utilization among Women of Reproductive Age in Choma District, Zambia.
Miyoba Hangoma
NMCZ
Miyoba Hangoma
NMCZ
DOI: https://doi.org/10.55320/mjz.52.1.613
Keywords:Cervical cancer, screening, Utilization, Determinants, women of reproductive age
ABSTRACT
Background
Cervical cancer is a significant global health challenge, ranking as the fourth most prevalent cancer and the fourth leading cause of cancer-related death among women worldwide. Zambia has one of the highest cervical cancer incidence and mortality rates due to under-utilization of cervical cancer screening services (CCSS).
Objective
The study aimed to assess the determinants of cervical cancer screening utilization among women of reproductive age in Choma District, Southern Province, Zambia.
Methods
A non-interventional, quantitative analytical cross-sectional study was conducted among 384 respondents using systematic random sampling techniques. Data was collected using a structured questionnaire and analyzed with SPSS version 26 using chi-square tests and binary logistic regression.
Results
The largest group of participants were aged 26-36, making up 52.6% (202) of the total, with a screening rate of 68.8% (139). The average age was 30.9 years (±7.75 years). The study found a cervical cancer screening rate of 66.9% among participants.
Employment was significantly associated with lower odds of CCS utilization (aOR = 0.62, 95% CI: 0.39 - 0.98, p = 0.041), while the provision of information by healthcare providers about CCS and prevention was significantly associated with higher odds (aOR = 1.60, 95% CI: 1.02 - 2.50, p = 0.040).
The availability of CCS services showed a significant association (uOR = 1.77, 95% CI: 1.05 - 2.99, p = 0.031), but this became non-significant after adjustment (aOR = 1.67, 95% CI: 0.97 - 2.89, p = 0.064).
Conclusion
The study found a 66.9% CCS rate, with employed women less likely to participate. Workplace-based screening programs and employer collaboration with health authorities are recommended to boost participation, along with promoting CCS awareness through wellness programs.
INTRODUCTION
Cervical cancer poses a significant public health challenge globally among other cancers; it is the highest burden of cancer with a high prevalence rate (23%), followed by Kaposi’s sarcoma (16%), Prostate cancer (11.2%), Breast cancer (7%), and esophagus cancer (3.7%).[1] It is particularly prevalent in Zambia, where it is the most common cancer among women aged 15 to 44.[2][3]
Cervical cancer poses a significant health burden in Zambia, with approximately 3,161 new cases diagnosed each year and a mortality rate of 1,904.[4] According to the Cancer Disease Hospital in Zambia, the annual report for 2021 indicated that cervical cancer was the leading cause of death among the top ten adult cancers, accounting for 156 deaths, or 30% of the total. In 2022, the mortality rate from cervical cancer rose to 205 deaths, representing 33% and affirming its position at the top of the list of adult cancers.[5]
The World Health Organization (WHO) launched the 90-70-90 global strategy to address the urgent issue of cervical cancer, specifically targeting low- and middle-income countries like Zambia. This strategy aims to achieve high vaccination coverage, increased screening rates, and improved treatment accessibility by 2030.[6] In Zambia, the National Cervical Cancer Prevention Programme (CCPPZ) was established in 2006 and focused on expanding access to and utilization of cervical cancer screening services.[7] WHO estimates that between 2019 and 2023, Zambia will provide 2,275,621 screening services to women aged 15 to 59 who are HIV-positive, as well as to women aged 25 to 59 who are HIV-negative. This initiative aims to achieve a nationwide coverage rate of 65%.[6] However, despite these efforts to enhance access to screening services, the actual utilization of these services remains low.
A 2017 study by the Centre for Disease Control found that only 2 out of 30 Zambian women used cervical cancer screening services due to barriers like limited healthcare access, social stigma, and perceived low risk.[8] Additionally, 21% of men disapproved of screening, with some preventing their wives from participating.[9]. In Choma District, which has 60,344 women of reproductive age[10], only 11,357 (18.8%) were screened for cervical cancer between 2018 and 2021.[11] Despite awareness campaigns, the reasons for this low utilization are poorly understood.Maree and Banda[12] identified socio-demographic factors such as geographical location and employment as barriers to screening. Negative attitudes from healthcare workers and a lack of public confidence in the health system also contributed to the issue.[13] Studies indicated that low education, older age, and distance to facilities were linked to advanced cervical cancer stages due to non-attendance at screenings.[13]
Therefore, this study aimed to assess the determinants of cervical cancer screening utilization among women of reproductive age in Choma District, Southern Province, Zambia. The findings from this study will guide relevant health facilities in developing plans and strategies to address observed gaps in cervical cancer screening among women of reproductive age and improve service quality.
MATERIALS AND METHODS
This study employed a quantitative analytical cross-sectional design conducted in selected health facilities across the Choma District. The study population consisted of women of reproductive age (15 to 49 years) who were available during the data collection phase. The sample size of 384 women was determined using the Cochran formula. The formula for calculating the sample size (Cochran formula) (Using prevalence formula):
n = Z2 ∙ P ∙ (1 – P)
d2
Where, n= This is the estimated sample size, d= Degree of error or the absolute precision ±5% p= Proportion of women who are not utilizing CCSS= 50%, Z= standard normal variant at 95% confidence level (CL) = (1.96), N = 384. Using the prevalence of cervical cancer screening from previous studies.
The prevalence of cervical cancer screening utilization, established at 44.8 percent in a study conducted by Daka et al.[15] in Kitwe, Copperbelt Province, Zambia, was employed as a reference point for this research, N = 380. Therefore, the population proportion sample calculated was considered since it calculated a larger sample was N = 384. Probability sampling was utilized to prevent bias, employing a systematic random sampling technique based on the average daily clinic attendance of women of reproductive age (15-49 years) for 60 days. The inclusion criteria encompassed all women of reproductive age from 15 to 49 years who were present during data collection and were residents of Choma district. Additionally, all women of reproductive age who provided consent to participate in the study during the data collection period were included. Exclusion criteria comprised participants with severe physical illness or mental incapacity. This included individuals who were severely ill and unable to provide coherent responses, as well as those with mental health conditions that hindered their ability to fully comprehend and engage in the study.
A structured questionnaire adapted from related literature[16] was utilized for data collection because all respondents were asked the same set of questions in the same order, reducing interviewer bias (where the interviewer might influence responses) and response bias (where respondents may give answers based on how questions are phrased). Content and face validity were ensured by soliciting suggestions from experts, advisers, and lecturers to assess its relevance, clarity, and consistency with the study. These individuals reviewed the questions to ascertain whether they would elicit the desired responses from the study population regarding the variables to be measured. The reliability and internal consistency of each question were confirmed through a reliability test. The items were measured with a Cronbach's alpha ranging from 0.83 to 0.92. These scales were adapted from peer-reviewed studies, such as that of Nigussie.[17]
A pilot study was conducted at Choma General Hospital to test the questionnaire's efficacy and to ensure that the questions were clear, unbiased, relevant to the target population, and measured the intended constructs. This pilot study involved participants who met the criteria for the study sample. The sample size for the pilot study was 10% (38) of the total study sample.
The data was analyzed using the SPSS computer package version 26 for Windows. Chi-square and Fisher’s exact tests were employed to assess associations between short-term outcomes and independent variables at a significance level of five percent. Univariable and multivariable binary logistic regression was utilized to determine the effect of independent variables on the utilization of cervical screening services. A backward stepwise approach guided by various model-fit statistics was adopted for model estimation. The study employed a significance level of 5%, with p-values of 0.05 or less considered statistically significant, leading to the rejection of the null hypothesis.
RESULTS
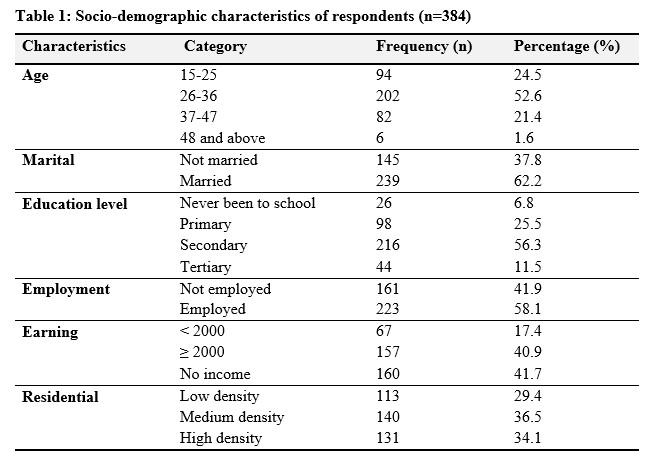
As shown in Table 1; out of 384 participants, the majority were within the 26-36 age group, comprising 52.6% of the sample, followed by the 15-25 age group at 24.5%. Participants aged 37-47 represented 21.4% of the sample, while those aged 48 and above constituted a smaller proportion at 1.6%. The majority of participants were married (62.2%), while 37.8% were not married. Regarding education level, the highest proportion of participants had secondary education (56.3%), followed by primary education (25.5%), with a smaller percentage having tertiary education (11.5%), and the lowest proportion having never been to school (6.8%). Employment status showed that 58.1% of participants were in employment, while 41.9% were not employed. The majority of participants (41.7%) reported having no income, while 40.9% earned an income of 2000 or more, and 17.4% earned less than 2000. Lastly, residential area distribution showed that 36.5% resided in medium-density areas, followed closely by high-density areas (34.1%), with low-density areas having the smallest proportion of participants (29.4%).
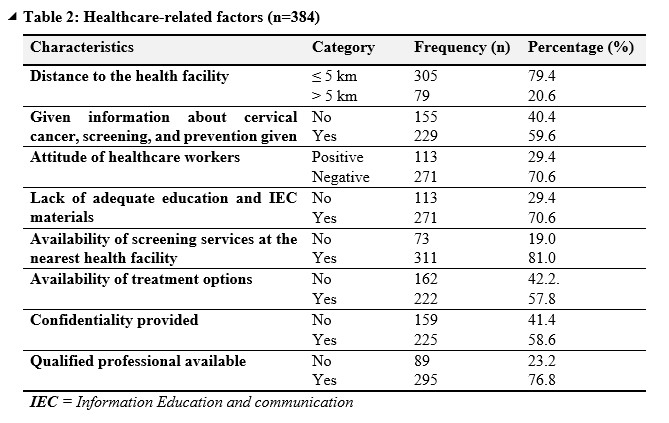
Table 2 indicates that; distance to the health facility revealed that a majority of respondents, comprising 79.4%, lived within a 5-kilometer radius of a health facility, while 20.6% resided farther than 5 kilometers away. Concerning education and awareness, a significant portion of participants, representing 40.4%, reported not receiving information about cervical cancer screening and prevention, while 59.6% indicated they had received such information. Attitudes towards healthcare workers were predominantly negative, with 70.6% of respondents expressing negative sentiments compared to 29.4% with positive perceptions. Lack of adequate education and information, as well as insufficient Information Education and Communication (IEC) materials, were reported by 70.6% of participants. Despite this, the majority (81.0%) reported the availability of screening services at their nearest health facility. Similarly, 57.8% indicated the availability of treatment options, although 42.2% reported the absence of such options. In terms of confidentiality and professionalism, 58.6% reported receiving confidentiality, and 76.8% noted the presence of qualified professionals at health facilities.
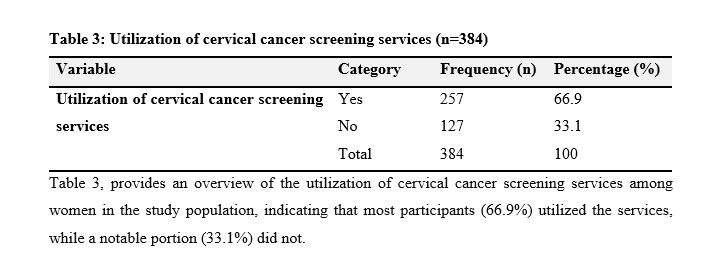
Table 3, provides an overview of the utilization of cervical cancer screening services among women in the study population, indicating that most participants (66.9%) utilized the services, while a notable portion (33.1%) did not.
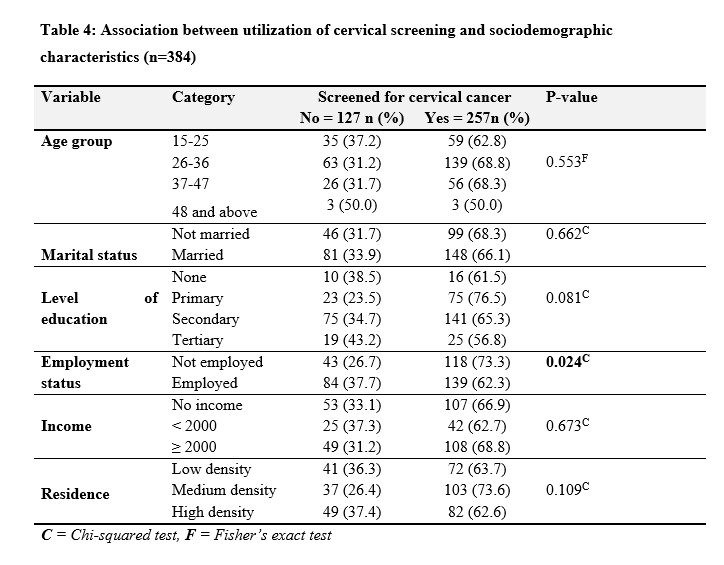
Table 4 shows no significant difference in screening rates among age groups (p = 0.553). Marital status did not significantly influence screening uptake (p = 0.662), while education level approached statistical significance (p = 0.081), with higher screening rates among individuals with primary and tertiary education. Employment status was significantly associated with screening utilization (p = 0.024), with 37.7% of employed individuals screened. Income level and residence did not show significant differences in screening rates (p = 0.673 and p = 0.109, respectively). The findings suggest that age, marital status, income level, and residence have no significant impact on cervical cancer screening utilization.
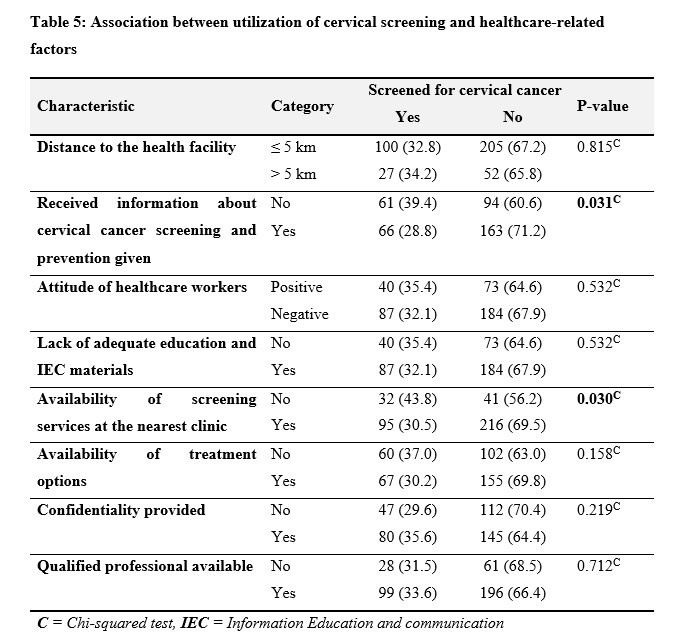
The study findings in Table 5 on cervical cancer screening utilization among different contextual factors were summarized. Distance to the health facility did not show a significant association with screening rates (p = 0.815), with 32.8% of individuals living within 5 kilometers and 34.2% living farther than 5 kilometers undergoing screening. However, receiving information about cervical cancer screening and prevention significantly influenced screening behavior (p = 0.031), with 28.8% of those informed opting for screening compared to 39.4% of those not informed. The attitude of healthcare workers and the availability of education and Information Education and Communication (IEC) materials did not exhibit significant associations with screening rates (p = 0.532). Conversely, the availability of screening services at the nearest clinic was significantly associated with screening utilization (p = 0.030), with 30.5% of individuals having access to screening services undergoing screening compared to 43.8% without such access. However, the availability of treatment options did not show a significant association with screening rates (p = 0.158). Confidentiality provided during the screening process did not significantly influence screening behavior (p = 0.219), nor did the presence of qualified professionals at health facilities (p = 0.712).

In the univariable analysis shown in Table 6, no significant associations were observed between age groups and Ca Cx screening utilization. However, in the multivariable analysis, age groups still did not show significant associations with Ca Cx screening utilization after adjusting for other variables. Regarding employment status, in the univariable analysis, being employed was associated with lower odds of Ca Cx screening utilization than being unemployed (unadjusted odds ratio [uOR] = 0.60, 95% CI: 0.39 - 0.94, p = 0.025). This association remained significant in the multivariable analysis (adjusted odds ratio [aOR] = 0.62, 95% CI: 0.39 - 0.98, p = 0.041). Monthly earning levels did not significantly correlate with Ca Cx screening utilization in univariable or multivariable analyses. Availability of cervical cancer screening (CCS) services was significantly associated with screening uptake in the univariable analysis (uOR = 1.77, 95% CI: 1.05 - 2.99, p = 0.031); however, this association became marginally non-significant in the multivariable analysis (aOR = 1.67, 95% CI: 0.97 - 2.89, p = 0.064). Similarly, the provision of Information Education and Communication (IEC) on cervical cancer screening was significantly associated with Ca Cx screening utilization in both univariable (uOR = 1.60, 95% CI: 1.04 - 2.47, p = 0.032) and multivariable analyses (aOR = 1.60, 95% CI: 1.02 - 2.50, p = 0.040). Other variables including age, monthly earnings, treatment availability, and confidentiality provision did not show significant associations with Ca Cx screening utilization in either analysis.
DISCUSSION
The study included 384 participants, with the majority (202) in the age range of 26-36 years, accounting for 52.6% of the total participants. Of these, 68.8% had undergone cervical cancer screening. The findings indicated that participants in the 26-36 age group were 1.24 times more likely to undergo cervical cancer screening compared to those aged 15-25 years. Women aged 37-47 years were 1.33 times more likely to be screened than those in the 15-25 age group. Notably, cervical cancer screening varies among different age groups. A study conducted by Dozie et al.[18] reported a higher prevalence of cervical cancer screening among participants aged 25-29 years (17.2%) compared to other age groups, suggesting that the likelihood of screening increases with age. These findings are also consistent with Nthiga's study [19] which found higher rates of cervical cancer screening among women aged 25-34 years. Similarly, Gebremariam [20] found that women in the 30–39 and 40–49 age groups were two and four times more likely to use cervical cancer screening, respectively. This may be because older women are at a higher risk of developing cervical cancer compared to younger women. Additionally, as they are more likely to be sexually active, they may be more aware of the importance of screening [21]. However, contrary to these findings, Gimba et al. [22] discovered that women aged 23 had higher screening rates, possibly due to earlier exposure to sexual activity. The differences in these studies may be due to cultural or geographical factors that affect when and how people get cervical cancer screening. For example, early sexual activity, as mentioned by Gimba et al. [22] might be more common in some areas, which could lead to higher screening rates among younger women in those regions.
However, the study found no strong statistically significant association between age and cervical cancer screening utilization (P = 0.553). While the likelihood of cervical cancer screening generally increased with age, this study found no strong statistical evidence to support age as a significant determinant of screening utilization. These findings imply that while cervical cancer screening rates tend to rise with age, age itself is not a strong factor influencing screening utilization. This indicates that other variables, such as education, healthcare access, or awareness, may be more important in determining whether individuals are screened.
The greater proportion of participants were married 239 (62.2%). This is similar to Mwangelwa’s study [23] which found over 64% of her sample, were married. This could be because the majority age group in this study was 26 - 36 since this age category consists of adult members of society who by their age society require them to be married. This could also be attributed to the fact that the participants were drawn from women who were available at the health facility during the data collection period in various clinics. Therefore, married women have more services they can utilize at the health center than unmarried women do. The study indicated no significant difference in screening rates between married and unmarried participants (p-value = 0.662). Both groups had similar percentages of being screened. On the contrary, Kaso et al. [24] also examined the factors associated with the likelihood of cervical cancer screening in women aged 20–39. It was reported that, compared to unmarried women, married women were 2.4 times more likely to have received screening for Ca Cx. Another study conducted by Che, et al. [25] found that married women had better CCSS than unmarried women (HR: 0.892, 95% CI: 0.850–0.937) by Cox regression analysis implying that marriage was a good prognostic factor of cervical cancer. Several studies have also reported this positive association between marriage and adherence to cervical cancer screening guidelines across all age groups [26]–[30]. The explanation was that married women may be at greater risk of contracting Human Papillomavirus (HPV) due to their increased exposure to sexual intercourse. Additionally, as women age, their risk of contracting HPV increases, which may explain why they are more likely to visit health facilities. The possible explanation for this discrepancy could be cultural attitudes towards marriage and healthcare access, in some cultures married women may have more encouragement or support to seek healthcare [31]. Therefore, systemic, personal, and cultural barriers as well as the lack of decision-making guidelines can be contributors to disparities in cervical cancer screening. However, a study by Aynalem [32] indicated that marital status was also one of the significant predictors for the utilization of cervical cancer screening. This study showed that women who were single/divorced/widowed were 3.414 times more likely to utilize cervical cancer screening as compared with married women (AOR:3.414, (95%CI:1.299,8.972). These results were supported by the study done in Thailand by Visanuyothin [33]. The possible explanation for this might be single women are more likely educated since they might be young and divorced women or widowed women are more likely aged and increasing risk with women’s age leads the women to have more interest in visiting health facilities. The highest number of participants resided in a medium-density residence 140 (36.5%) and the lowest number of participants resided in low-density residences 113 (29.4%). Of those who resided in a medium-density place, 103 (73.6%) had undergone cervical cancer screening and only 72 (63.7%) of those who resided in a low-density area were screened for cervical cancer. These findings were consistent with Mpachika et al. [34] and Kagika [35] who found that low uptake of cervical cancer screening was associated with socio-demographic factors such as age; religion; ethnicity, and place of residence. The place of residence was related to accessibility issues, as even when an individual desires to undergo screening, the distance to the facility where the service is provided often undermines the purpose due to the time and effort required from the patient.
A larger proportion of the participants accounting for 223 (58.1%) were employed either in formal employment or informal employment while the rest 161 (41.9%) were not in any type of employment. The findings reviewed that being employed was associated with lower odds of Ca Cx screening utilization compared to being unemployed (uOR= 0.60, 95% CI: 0.39 - 0.94, p = 0.025). This association could be due to employed individuals having less flexibility in their schedules, making it more difficult to access healthcare services like cervical cancer screening (CCS). Additionally, employed individuals may prioritize work and other immediate responsibilities over preventive healthcare, such as CCS. High stress levels associated with work might also contribute to neglecting regular health check-ups. However, the observed association is inconsistent with a study conducted in Harare, Zimbabwe [36] which found no significant association between employment status and CCS. In contrast, the findings of the study align with those of Bukirwa et al. [37], who reported that employed women had a 13% higher likelihood of undergoing CCS. This was attributed to certain jobs or sectors offering more flexibility in scheduling, enabling women to take time off for healthcare appointments without losing income and greater financial stability. While both studies show some connection between employment and cervical cancer screening, the different results are likely due to contextual, demographic, and methodological differences. The study argues from the perspective that being employed may confer greater financial stability, reducing economic barriers to accessing screening services and enabling women to initiate CCS earlier than their counterparts who are unemployed. In some instances, being employed decreases women’s access to CCS due to commitments, particularly for those in formal or informal sectors with fixed hours or multiple jobs, which may limit their availability during clinic hours.
The study indicated that the majority of participants (33.1%) who had no source of income did not screen for cervical cancer. These findings were similar to Ndateba et al.[38] who found that the participants with lower economic status were less likely to utilize cervical cancer screening services. Furthermore, the odds of cervical cancer screening uptake among participants with high income per month were 5.7 times higher compared to those with no or less income per month (OR:5.661;95%CI:.1.878-17.057, p =.002). Additionally, current occupations with governmental and private employees were four and three times more likely to utilize the screening service [20] This was attributed to women with no or lower income struggling with their daily basic needs and facing multiple challenges in accessing health care services. Thus, underscoring the significance of economic status as a significant predictor of age at first screening for cervical cancer [39].
The findings of the present study indicated that the magnitude of cervical cancer screening was 66.9%. The study findings are higher than those found by Daka[15] where out of the 210 women enrolled in the study, only 94 (44.8%) reported having ever been screened for cervical cancer in their lifetime. In addition, higher than those in other low- and middle-income countries; for example, 39% in Botswana [40], and 25% in Tanzania [41] The possible explanation for this above-average cervical cancer screening utilization could be due to the high level of awareness and availability of specificity of national cancer prevention and control strategies in Zambia. However, despite having a 66.9% utilization of CCSS by participants the independent variables had a greater influence on the utilization of CCSS.
The study findings revealed a significant association between participants who received Information, Education, and Communication (IEC) about cervical cancer screening and prevention (P= 0.031). Notably, 66 individuals (28.8%) underwent screening among those who received IEC, whereas only 61 individuals (39.4%) without access to such information were screened for cervical cancer screening. Similarly, the provision of IEC on cervical cancer screening was significantly associated with screening for cervical cancer in both univariable (uOR = 1.60, 95% CI: 1.04 - 2.47, P= 0.032) and multivariable analyses (aOR = 1.60, 95% CI: 1.02 - 2.50, (P= 0.04). The findings of the study are consistent with Dejene et al. [42] indicating that women who received information about cervical cancer screening and prevention were about 3.4 times more likely to use cervical screening than those who were not provided information about cervical cancer screening and prevention. This implies that if women are not given health education on cervical cancer screening and prevention they are less likely to go for cervical cancer screening. The findings were also consistent with the result of a study done in Dare Salam by Mugassa and Frumence [43], which indicated that the national health system factors influencing the early uptake of cervical cancer screening services include a poor flow of information from national to lower level, indicating poor dissemination of information about the importance of cervical cancer screening. Therefore, this entails that, any visit to health care facility by women provides a good opportunity to receive health education and increases the chances of exposure of service users to health information, which would in turn increase screening for cervical cancer.
Healthcare services as indicated by the findings of this research greatly influence utilization. Hence, health workers should strive hard to give information to women about cervical cancer screening and prevention as this could positively influence women to utilize screening services. Additionally, health workers can utilize media forums, and outreach clinics in communities to sensitize women about the importance of screening for cervical cancer. The study findings could also suggest that healthcare policies and interventions aimed at increasing cervical cancer screening rates should focus on educational initiatives on cervical cancer screening services.
The findings of the study also indicate that the provision of IEC materials did not result in a statistically significant difference in cervical cancer screening rates. Both groups, with and without IEC materials, had similar proportions of individuals screened for cervical cancer (35.4% and 32.1% respectively). This suggests that the presence or absence of IEC materials alone may not be a determining factor in influencing screening behaviors. Despite a slightly lower screening rate in the group provided with IEC materials, the difference was not statistically significant. The findings of the study also indicate that despite a slightly higher screening rate in the group of participants who indicated that healthcare workers had a positive attitude 87 (32.1%) than those who stated a negative attitude of health workers 40 (35.4%), the difference was not statistically significant (P>= 0.532). This suggests that the attitude of healthcare workers alone may not be a determining factor in influencing screening behaviors among women of reproductive age. The study also indicated that the provision of confidentiality did not lead to a statistically significant difference in cervical cancer screening rates. Despite a slightly higher screening rate in the group where confidentiality was provided 80 (35.6%) and 47 (29.6%) for those not provided with confidentiality, the difference was not statistically significant. These findings from this study are inconsistence with Mutyaba et al. [44] who found that the attitude of healthcare workers and confidentiality were statistically significantly associated with cervical cancer screening. The study indicated that the attitude of health personnel and the issue of confidentiality with medical information and privacy were some of the factors militating against screening for cervical cancer. This study was consistent with the findings of other studies like Julinawati et al.[45] that established that the inability to maintain the confidentiality of patient information, test results, rude behavior of some health personnel, and concerns about privacy during screening serve as barriers to the utilization of screening services. Some women consider the unfriendly attitude of some health personnel and concerns about medical information privacy as crucial factors in determining whether they would go for cervical cancer screening and treatment. These concerns may likely serve as barriers to utilizing cervical cancer screening and treatment and in turn have negative implications for the fight against this disease.
The study indicated that the availability of an effective cervical cancer screening program at the nearest health facility was significantly associated with undergoing cervical cancer screening (P = 0.03). A substantial proportion comprising 69.5% reported having cervical cancer screening services at their local health facility and had undergone screening compared to the 56.2% who reported the service as unavailable. However, the availability of treatment options, the presence of a qualified professional, and the distance to the health facility did not show significant associations with screening behavior. Similar findings were observed by Njuguna et al. [46] who identified barriers to the utilization of cervical cancer screening services, such as not knowing where to find such services, lack of availability of screening services, or being outside the catchment area of a healthcare facility providing screening services. Additionally, a majority of women noted time limitations and long waiting times at clinics as barriers [46]. Consistent with these findings, this study revealed that having a cervical cancer screening program at the health facility increased the likelihood of an individual opting for screening by 1.77 (95% CI 1.053-2.989).
Therefore, if cervical cancer screening services are unavailable, it can hinder women from undergoing cervical cancer screening. These findings underscore the importance of ensuring the availability and accessibility of screening services to encourage cervical cancer screening among the population.
The study results reveal that proximity to a health facility does not significantly affect the likelihood of undergoing cervical cancer screening (P = 0.815). Specifically, among women living within 5 kilometers of a health facility, 32.8% had undergone screening, compared to 34.2% among those living more than 5 kilometers away. Additionally, Wangechi et al. [47] found that distance to the health facility did not correlate significantly with previous participation in cervical cancer testing, as nearly 70% of respondents reported no prior participation regardless of distance. However, women without perceived distance issues were 4.4 times more likely to utilize cervical cancer screening services (AOR=4.41, 95% CI=2.53–9.41) [48].
Moreover, perceived distance from the screening health facility emerged as a significant predictor for cervical cancer screening uptake in this study. Women aged 15 years or older with easy access to health facilities for screening were 4.45 times more likely to undergo screening than those facing longer travel distances (AOR=4.41, 95% CI=2.53–9.41). [48]
These findings are consistent with those of Nyamambi [9], whose study in Zimbabwe highlighted long distances to health facilities offering cervical cancer screening as a barrier to utilization. Thus, although proximity to health facilities may not directly impact screening for cervical cancer, perceived distance plays a significant role in utilization rates. Ensuring easy access to screening facilities is crucial for improving cervical cancer screening rates among women. This suggests that establishing more screening and treatment facilities near residential areas could enhance the uptake of screening and treatment services.
CONCLUSION
The study highlights the importance of targeted interventions, such as enhancing healthcare worker communication and increasing accessibility to screening services, to improve cervical cancer screening rates in Choma District. Additionally, the study emphasizes the positive impact of Information, Education, and Communication (IEC) on cervical cancer screening. Expanding educational programs that raise awareness about cervical cancer and the benefits of screening is essential. Such programs should be tailored to different demographics, ensuring that women of reproductive age in Choma District receive accurate and accessible information through various platforms, including health centers, media, and community outreach.
RecommendationsFollowing the study's findings, several recommendations have been proposed regarding the cervical cancer screening program in Zambia:
- Promoting cervical cancer screening should develop messages that can be easily understood by individuals with no education or only a primary education level. This approach aims to ensure that everyone can appreciate the importance of cervical cancer screening services.
- The Ministry of Health should enhance financing for programs to prevent cervical cancer. This includes conducting mass screening for cervical cancer at every health facility and within health facility catchment areas.
- Health promotion messages about cervical cancer screening should utilize a hotspot approach. This involves targeting women in various settings such as churches, markets, schools, and workplaces to ensure a broader reach.
- Information, Education, and Communication (IEC) efforts emphasizing the importance of cervical cancer screening services should be integrated into the Maternal and Child Health care package. This integration ensures that women who were unable to be screened during pregnancy can undergo screening as soon as they become eligible.
By implementing these recommendations, the cervical cancer screening program in Zambia can be enhanced to reach a wider audience and effectively reduce the burden of cervical cancer in the population.
AcknowledgmentFunding for this study was supported by the Fogarty International Center of the National Institutes of Health, U.S. Department of State’s Office of the U.S. Global AIDS Coordinator and Health Diplomacy (S/GAC) and the President’s Emergency Plan for AIDS Relief (PEPFAR) under the Award Number R25 TW011219 under the project title: Strengthening Health Professional Workforce Education Programs for Improved Quality Health Care In Zambia (SHEPIZ) Project. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Firstly, I thank God for seeing me through my entire academic journey.
Ethical ConsiderationEthical approval was sought from The University of Zambia Biomedical Research Ethics Committee (UNZABREC), and then permission to conduct the study was requested from the National Health Research Authority and Choma District Health Office.
Conflict of InterestThe author reports no conflicts of interest in this work.