Pattern and Quality of Antimicrobial Prescribing in a Nigerian Tertiary Hospital: Report of a Longitudinal Surveillance Cautioning on Increasing Threats to Antimicrobial Resistance.
Abayomi Fadeyi
Medical Microbiology & Parasitology Department, University of Ilorin, Ilorin, Nigeria
Olalekan Ayodele Agede
Pharmacology & Therapeutics Department, University of Ilorin, Ilorin, Nigeria
Sunday Adedeji Aderibigbe
Community Health & Epidemiology Department, University of Ilorin, Ilorin, Nigeria
Sherifat Tinuke Suleiman
Medical Microbiology & Parasitology Department, University of Ilorin, Ilorin, Nigeria
Olufemi Ademola Lawani
Medical Microbiology & Parasitology Department, University of Ilorin, Ilorin, Nigeria
Mariam Kehinde Sulaiman
Medical Microbiology & Parasitology Department, University of Ilorin, Ilorin, Nigeria
Moses Ojonyene Kadiri
Medical Microbiology & Parasitology Department, University of Ilorin Teaching Hospital, Ilorin, Nigeria
Jibril Imran
Medical Microbiology & Parasitology Department, Nile University, Abuja, Nigeria
Ademola Amos IDOWU
Chemical Pathology Department, Ekiti State University, Ado-Ekiti, Nigeria
Abdullah Dasilva Yussuf
Behavioural Science Department, University of Ilorin, Ilorin, Nigeria
Ann Versporten
Laboratory of Medical Microbiology, Vaccine & Infectious Diseases Institute, University of Antwerp, Belgium
Herman Goossens
Laboratory of Medical Microbiology, Vaccine & Infectious Diseases Institute, University of Antwerp, Belgium
Ines Pauwels
Laboratory of Medical Microbiology, Vaccine & Infectious Diseases Institute, University of Antwerp, Belgium
Oyinlola Omoniyi Oduyebo
Medical Microbiology & Parasitology Department, University of Lagos, Lagos, Nigeria
DOI: https://doi.org/10.55320/mjz.51.4.566
Keywords:Antimicrobial prescribing pattern, Antimicrobial prescribing quality, Point prevalence, Nigeria
ABSTRACT
Background: Indiscriminate use of antimicrobials is threatening their effective use owing to resistance. This study aims to describe the pattern and quality of antimicrobials prescribing at the University of Ilorin Teaching Hospital, Ilorin, Kwara State, Nigeria (UITH) using the five-year data from the Global-Point Prevalence Surveillance (G-PPS).
Method: G-PPS, a web-based software, was used among inpatients from 2017 to 2022 according to the protocol designed at the University of Antwerp, Belgium. Data collected using the standardised questionnaire were inputted, cleaned and submitted with the software which gives auto-analysed results immediately.
Result: A total of 783 patients and 1281 antimicrobial prescriptions were studied. The 5-year mean overall antimicrobial prevalence was 79.8% and 71.6% for Paediatric and Adult patient population respectively. Overall, there were more intravenous prescriptions (75.9%) than other routes. Polypharmacy with multiple antibiotics use for a single diagnosis (57.1%) and patient (57.6%) were prevalent. The “Access” (51.0%) category of antimicrobials were equally often prescribed as the “Watch” (48.2%) with few “Not Recommended” (0.8%). Most prescriptions were empirical. Indication for antibiotics prescription, and the stop/review date were poorly documented. Antimicrobial prescribing guidelines, such as antibiogram, were not available hence the failure of compliance to any guideline.
Conclusion: Antimicrobial prevalence in this study was high, and the quality of prescribing was also unsatisfactory. This requires intervention at many levels, focusing on prescribers, hospital administrators, healthcare policy makers and government. Failure of modulating to ensure rational antimicrobial prescribing may constitute a threat of returning to the casualties of the ‘Pre-antimicrobial Era’.
INTRODUCTION
The ability of microorganisms to become resistant to the major therapies used against them has long been recognized, yet becoming increasingly apparent.[1 ,2] A recent report has shown that Antimicrobial resistance (AMR) is a leading cause of death worldwide, higher than HIV/AIDS or malaria.[3] More than 1.2 million people are dying as a direct result of antibiotic-resistant bacterial infections across the world.[3] It has also been reported that Antimicrobial-resistant infections claims at least 50,000 lives each year across Europe and the US alone, with many hundreds of thousands more dying in other areas of the world.[4] In Western Sub-Saharan Africa, in comparison to other regions of the world, the highest burden of AMR was recorded with an estimated 27.3 deaths per 100, 000 directly attributable to AMR while 114.8 deaths were associated to AMR. Has the highest attributable and associated burden of AMR of about 27·3 deaths per 100,000 and 114·8 deaths per 100,000.[3]
Many factors are responsible for the emergence and spread of AMR.5 Overuse and misuse of antimicrobial agents constitute a major factor.[6] The extent of overuse and misuse of antimicrobial agents, in a community, determines the selection pressure for a resistant mutant pathogenic microorganism.[7] The strength of selection pressure on the other hand is the most important parameter contributing to the complexity of antibiotic resistance evolution and subsequent spread.[8,9]
In our present study, we plan to report the prescribing rates of antimicrobials and the lapses in prescribing using the five-year data from the Global-Point Prevalence Surveillance (G-PPS). It is believed that factors such as the quantity and quality of antimicrobial prescribing are contributory to the selection pressure for resistant mutant pathogenic microbes in any community and as such, the emergence and spread of resistance. This report, therefore, is aimed at providing useful data for policy makers in our setting towards appropriate antimicrobial prescribing and improved patient health.
The G-PPS standardised method has been developed by researchers at the University of Antwerp and funded by BioMérieux. Detailed information on the method of the G-PPS is described elsewhere.[10] It is primarily a web-based data collection tool for monitoring the rates of antimicrobial prescribing and resistance to infections in hospitalised patients. Researchers are encouraged to participate in using the tool to generate global data that can be used to address the challenge of AMR across the world. Researchers at the University of Ilorin Teaching Hospital, Ilorin, Kwara State, Nigeria, had enrolled into the G-PPS program since 2017 and have been conducting surveillance at least once yearly except for year 2020 when it was difficult to do so owing to COVID-19 pandemic. This is therefore a 5-year longitudinal surveillance report of G-PPS conducted at our centre between 2017 and 2022.
METHODOLOGY
Study site:
The longitudinal surveillance of antimicrobial use and prescribing pattern was conducted at UITH, a tertiary health institution located in Ilorin, Kwara State, Nigeria.
UITH bed space capacity is 650. Even though it is a tertiary health centre, it also serves as a primary and secondary health facility. It receives referrals from the neighbouring states of Oyo, Osun, Ekiti, Kogi and Niger. The hospital has six main ward types which includes Adult Medical Ward (AMW); Adult Surgical Ward (ASW); Paediatrics Medical Ward (PMW), Paediatrics Surgical Ward (PSW), Adult Intensive Care unit (ICU), Neonatal Intensive Care Unit (NICU).
Study design and setting
G-PPS, a web-based software for data-entry, validation and reporting was used to conduct a longitudinal survey on antimicrobial use and prescribing pattern at UITH. The web-based tool has capacity to collect information on hospitalised patient such as age, gender, ward of hospitalisation and data on antimicrobial therapy. Data collected about antimicrobial use includes antimicrobial agent type(s), number of doses per day, route of administration, documentation of indications for treatment, microbiological investigation done and results, compliance to any antimicrobial prescribing guidelines (AMPG) such as periodically locally published antibiograms and availability of a local empiric prescribing guideline as well as records of the stop/review date. The information was extracted majorly from patients’ medical records supplemented with nurses and physician’s interview. All patients on admission as at 8am on survey day in each ward of the hospital were captured and constitutes the denominator while those on at least one antimicrobial as at 8am on survey day represents the numerator. All patient information was collected with the aid of standardized data collection paper forms and subsequently inputted into the G-PPS website for analysis.
Data analysis
The G-PPS software has capacity for auto-analysis of data. Preliminary reports are therefore available on the G-PPS website instantly after the submission of inputted data. The data as inputted into the G-PPS software is convertible into excel spreadsheet and as such, further explored to generate additional reports after analysis. The reports for five (5) years (2017 – 2022) excluding 2020 were extracted and compared using descriptive statistics.
RESULTS
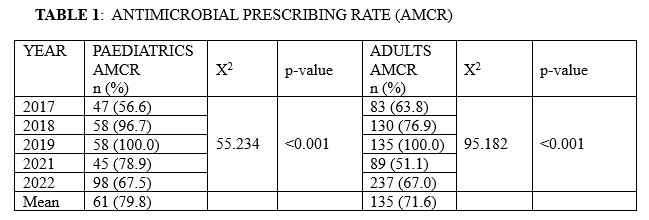
A total of 1, 680 patients comprising of 1,037 (61.7%) adults and 643 (38.3%) paediatric age group were studied over the 5-year period, from 2017 to 2022. There were 1281 antimicrobial prescriptions. The mean overall Antimicrobial Prescribing Rate was 79.8% and 71.6% for Paediatric and Adult patient population respectively (Table 1). The highest Antimicrobial Consumption Rate (AMCR) occurred in 2019 when all Paediatrics and Adult patients (100%) were on antimicrobials. Conversely, the lowest AMCR for Adult (56.6%) and Paediatric (51.1%) patients were observed in 2017 and 2021 respectively. The AMCR significantly changed over the study period for both Adults (p < 0.001) and Paediatric patients (p < 0.001). Less antifungals and antivirals for systemic use and drugs to treat TB were prescribed as against antibacterial agents and nitroimidazole derivatives which constituted the main prescriptions.
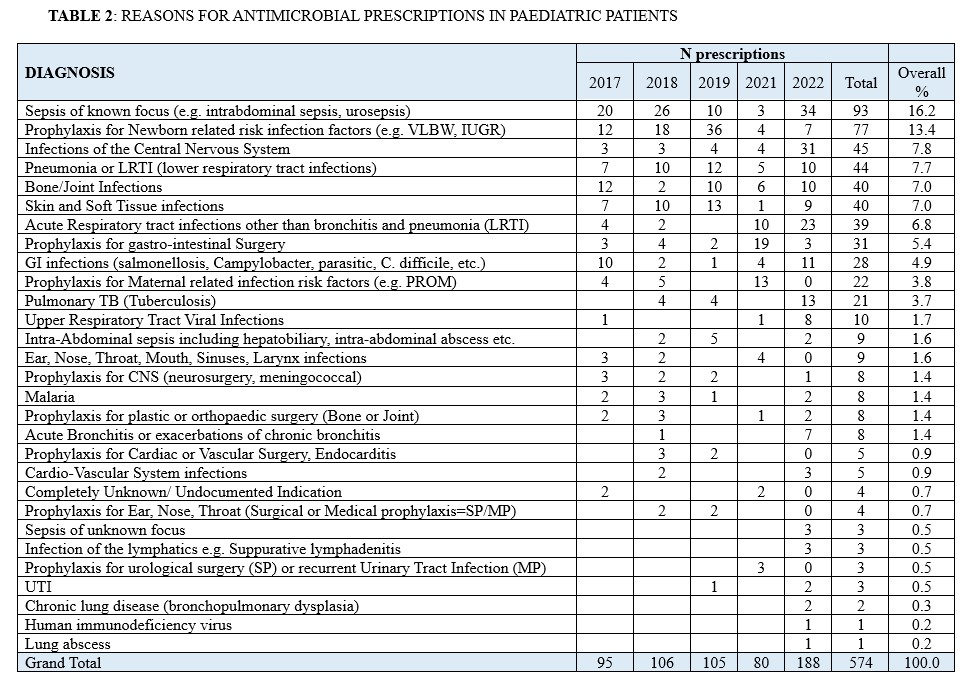
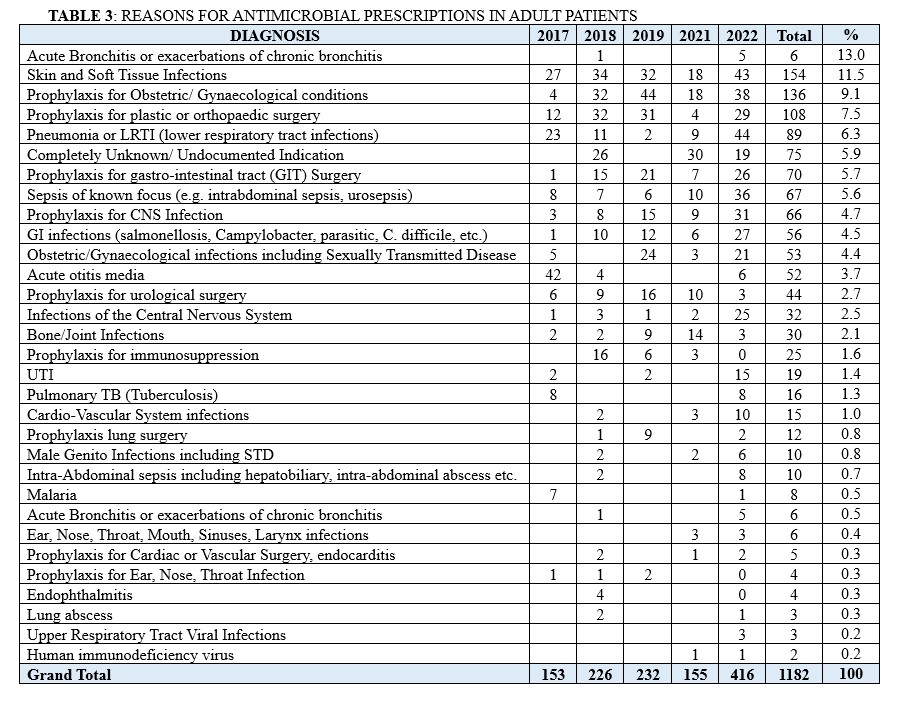
The commonest reason for antimicrobial prescription in Paediatric patients was Sepsis of known focus, totalling 93 prescriptions (16.2 %). This is followed by prophylaxis use for newborn related risk infection factors such as Very Low Birth Weight (VLBW) and Intrauterine Growth Restriction (IUGR) (n =77; 13.4%), Infections of the Central Nervous System (n=45; 7.8%), Pneumonia or LRTI (lower respiratory tract infections) (n = 44; 7.7%) and skin and soft tissue infections (n = 40; 7.0%) (Table 2). Conversely, in adult patients, skin and soft tissue infections emerged as the main reason for antimicrobial prescription, accounting for 13.9% (n =154). This is followed by prophylaxis for obstetric and gynaecological conditions (n =138; 12.5%), prophylaxis for plastic or orthopaedic surgery (n = 108; 9.8%), Pneumonia or LRTI (lower respiratory tract infections) (n=89; 7.9%) and prophylaxis for the gastro-intestinal tract (GIT) disorders (n =70; 6.3%). (Table 3)
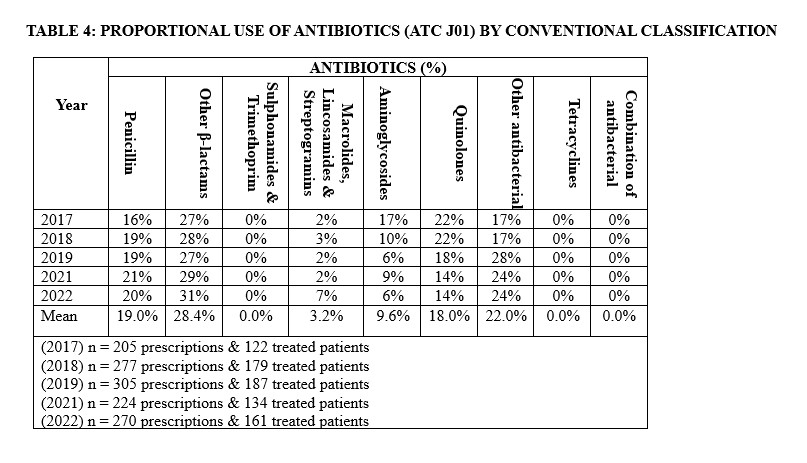
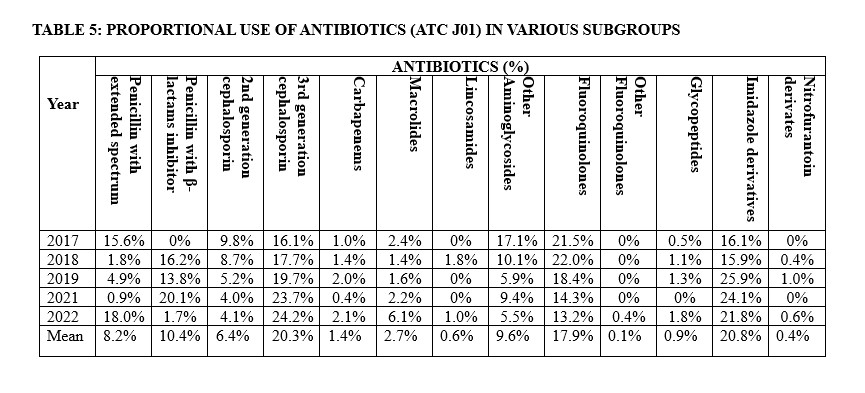
Other β-lactam antibacterial agents constituted the most prescribed in our hospital accounting for 28.4% (Table 4). Within this class, third-generation cephalosporins emerged topmost accounting for 20.3%. Considering prescription by antibiotics group (ATC J01), the imidazole derivatives, mainly metronidazole, were the most prescribed accounting for 20.8% of all prescriptions for both medical and surgical reasons, followed by third-generation cephalosporin and fluoroquinolones, accounting for 20.3% and 17.9% of the prescriptions, respectively in our hospital. (Table5).
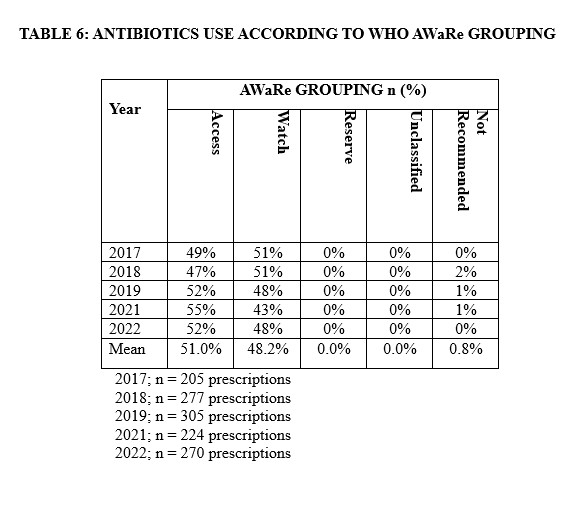
The prescription and thus rates of use of various antimicrobial agents significantly changed over the 5-year period of study. The prescription of extended-spectrum penicillin declined from 15.6% to 0.9% between 2017 and 2021, followed by an increase to 18.0% in 2022. However, Aminoglycosides showed a significant linear decline from 17.1% to 5.5% from 2017 to 2022. Concurrently, there was a consistent decline in the use of fluoroquinolones, penicillin with β-lactam inhibitors, and second-generation cephalosporins. Conversely, there was a steady increase in the utilization of imidazole-derived and third-generation cephalosporins. Based on the WHO AWaRe classification, most antibiotics (51.0%) prescribed belong to the “Access” category while 48.2% were of the “Watch” antibiotics. Only 0.8% of prescription fell under the category of “Not Recommended” (Table 6).
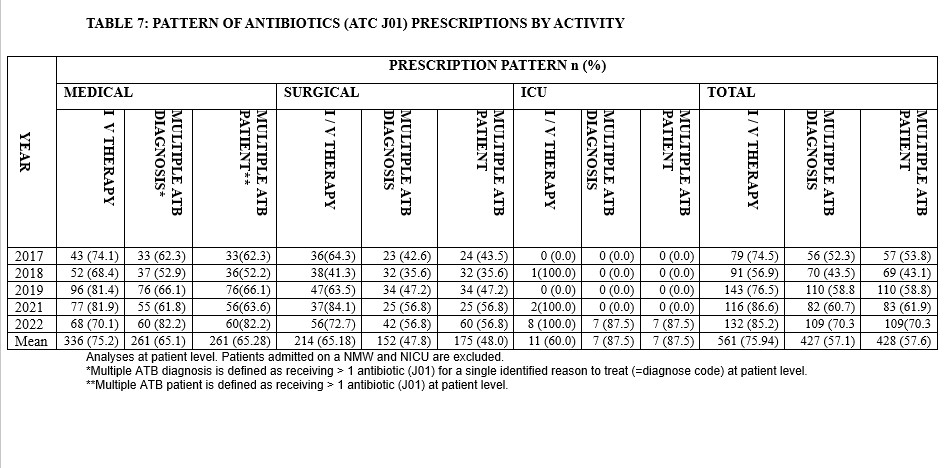
Over the period of study, there were more antibiotics prescribed intravenously (75.9%) compared to the oral route of administration. The prevalence of use of multiple antibiotics for a single documented diagnosis as well as calculated at patient level ranged from 52% to 82% (Table 7).
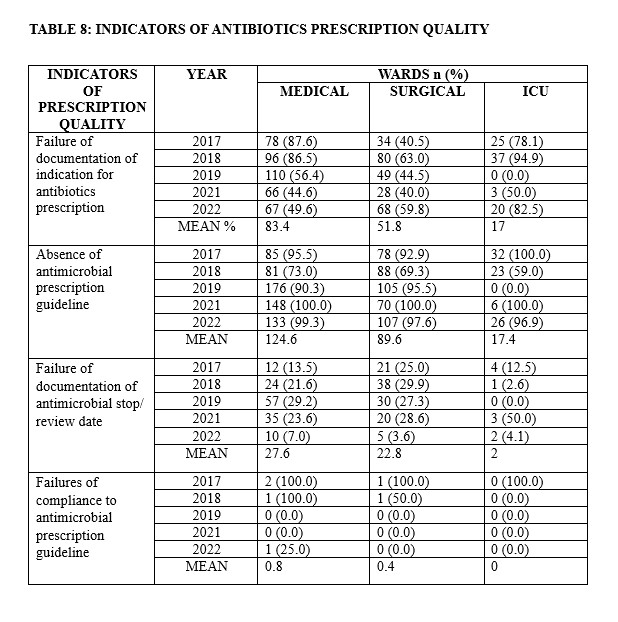
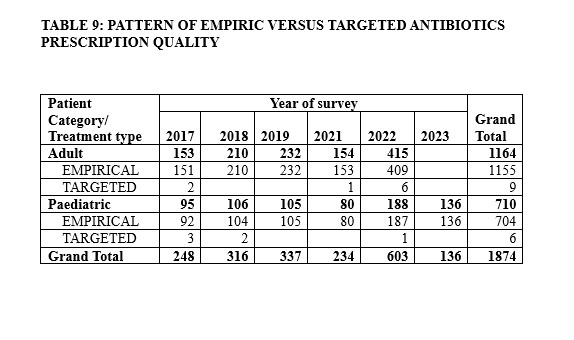
An overview of the antimicrobial prescription quality indicators is listed in Table 8. There were failures of documentation of indication for antibiotics prescription, failure of documentation of antimicrobial stop/review date documented in the patient hospital records, absence of antimicrobial prescribing guidelines such as antibiogram at prescription sites, and hence, non-compliance to any antimicrobial prescribing guideline across all hospital units. The proportion of targeted prescriptions were negligible as most prescriptions were empiric (Table 9).
DISCUSSION
The discovery and use of antimicrobial agents have changed the practice of medicine globally and led to a significant decrease in the morbidity and mortality associated with infectious diseases.11 However, antimicrobial resistance (AMR) emergence and spread remains a growing global challenge affecting mostly the developing countries including Nigeria. Nigeria has identified AMR as an emerging health threat deserving the generation of locally relevant data to guide evidence-based practice and interventions.[12] This study provides the first estimates of longitudinal human antibiotic prescribing for this centre from 2017 to 2022.
This study reported a high AMCR that changes over the years and was statistically significant in both paediatric and adult patients. This finding was like the ones reported by Ayukekbong et. al.[13] , Browne et. al.,[14] and Ekuma et. al.,[15] in their respective studies. This may be attributed to empirical treatment of cases of fever or suspected infection and the absence of functional Antimicrobial Stewardship Program (ASP) in our hospital. In 2020, Rabie et. al.,[16] did a study that evaluated the prescribing and dispensing practices using WHO standard indices and found that antibiotics were the most prescribed drug. There is robust evidence on the high use of antimicrobial (AM) agents in sub-Saharan African countries including Nigeria[17,18] which was attributed to high incidence of infections. β-lactam antibacterial were the most used class of antibiotics with ceftriaxone the leading agents. Lakoh et. al.,[19] reported amoxicillin-clavulanic acid as the most used antibiotics in their study which was similar to our finding.
A little above average of all the antibiotics prescribed in our study were from the WHO ‘access’ category, just as previously reported elsewhere.[20] This is a good clinical practice for our hospital which needs to be improved on as it still falls short of the 2023 recommendation by WHO. Sharma et. al.,[21] and Horumpende et. al.,[22] reported similar findings in their studies. The WHO AWaRe tool categorises antibiotics mainly into three group: the access, the watch, and the reserve group. This classification is of clinical significance when adhere to as it minimizes irrational prescription and the selective pressure for antimicrobial resistant phenotypes and their spread. The ‘access’ antibiotics as defined by WHO have narrow spectrum, with fewer side effects, reduced chances of antimicrobial resistance selection, and lower costs.[23] The bulk of antibiotics in our study were from this group which was in tandem with the WHO guideline. It is however important to note that the remaining percentage of antibiotics (48.2%) prescribed in this study were from the ‘watch group’. This was a worrisome finding as watch group of antibiotics carries a higher risk of promoting antimicrobial resistance and the chance of overuse is high. Some literatures had also reported the chances of overusing the watch group of antibiotics.[24,25] 24,25 The projection of WHO was that by 2023 at least 60% of all antibiotic prescriptions should be from the access group.[26]
Rational prescription and use of antibiotics, following a definitive diagnosis where possible, is a chief corner piece in antimicrobial stewardship. In this study it was found that most of the patients were treated empirically. Lakoh et. al.,[27] reported a similar finding in their study while Cox et. al.,[28] reported a different finding. It was also noted that most of the prescribed antibiotics were administered via the intravenous route which was contrary to the recommendation by the WHO. This practice has been reported to promote antibiotics resistance with the attendant problem of worsening morbidity and mortality[29] and as such prescribers in our hospital needs to be counselled and educated about the issue.
The pattern of prescribing antibiotics for patients in the assessed hospital wards revealed that for a single diagnosis in a patient, multiple antibiotics were prescribed. This practice was not justified and not in tandem with the recommendation by WHO. Multiple antibiotics use in a patient without justification may worsen the already identified increasing problem of antimicrobial resistance. Besides, patients affected with this practice may have issues with treatment compliance due to increased pill burden which ultimately affect treatment outcome. Maina et. al.,[30] and Desalegn et. al.,[31] in their studies on prescription practices reported the practice of polypharmacy and its attendant associated negative effects on treatment outcome that must be avoided by all medical practitioners.
Assessment of the indicators of antimicrobial prescribing quality identified some inadequacies over the period of study. These inadequacies occurred in all the wards though was predominant in the medical units due to the large number of patients that presented to medical outpatient department and medical emergency for treatment compared to the surgical wards and ICU with fewer patients. Similar unsatisfactory quality of antimicrobial prescribing has been reported by Kilipamwambu et. al.,[32] and Lam et. al.[33] The reasons for the unsatisfactory quality of antimicrobial prescribing such as failure of documentation of indication for antibiotics prescribing and their stop/ review dates, as recorded in the present study, is difficult to explain. These inadequacies of antimicrobial prescribing quality constituted significant prescribing errors which could give rise to poor compliance, drug-drug interaction, development of adverse drug reactions, and treatment failure especially from resistance. It is therefore pertinent to put mechanisms in place to checkmate unacceptable practise of antimicrobial agents’ prescribers in our locality.
It is hoped that rational antimicrobial usage will be encouraged at our centre as interventional strategy given the lapses identified in the current study. Such interventions are necessary to reverse growing global antimicrobial resistance scourge as precipitated by the indiscriminate use of antimicrobials. The approach will be to stimulate the adoption of all the principles of antimicrobial stewardship and make effective the stewardship committee in our setting. The multi-disciplinary involvement in the ASP will be motivated as it gives multiple opportunity for documentation of antimicrobial uses as well as checkmating point for prescribers. Our health facilities will be also stimulated to key into the ongoing national ASP activities in decreasing the wrong antimicrobial uses.
In conclusion, this research identified high antibiotics prescribing rates and unsatisfactory quality of antimicrobial prescribing which requires intervention at the levels of the prescribers, hospital administrators, healthcare policy makers and government. The WHO in 2011 had paraphrased “Antimicrobial resistance, no action today, no cure tomorrow”. Failure of modulating and ensuring rational antimicrobial prescription and use constitute a threat to returning to the casualties of the ‘Pre-antimicrobial Era’. Consequently, training, re-training and counselling of healthcare practitioners, especially the doctors and nurses, as well as enforcement of relevant legislation focusing on correct usage antimicrobials is strongly recommended in addition to institution of vibrant ASP in all health facilities of our setting.
ACKNOWLEDGEMENT
The authors acknowledge the management and all staff of the University of Ilorin Teaching Hospital, Ilorin (UITH, Ilorin) for their support during the surveillance that led to this publication.
Conflict of interest
Authors declare no conflict of interest
Source of funding
The Global Point Prevalence Survey is coordinated by the University of Antwerp, Belgium and sponsored through an unrestricted grant given annually by bioMérieux. However, UITH, Ilorin as a participating hospital did not receive any funding support directly for the surveillance.
REFERENCES
- Smith RD, Coast J. Antimicrobial resistance: A global response. Vol. 80, Bulletin of the World Health Organization. 2002.
- O’Neill J. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations, Review on Antimicrobial Resistance, Chaired by Jim O’Neill, December 2014. Review on Antimicrobial Resistance. 2016;(December).
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399(10325):629–55.
- Neill JO’. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations The Review on Antimicrobial Resistance Chaired. 2014;(December).
- Knobler SL, Lemon SM, Najafi M, Burroughs T. The Resistance Phenomenon in Microbes and Infectious Disease Vectors: Implications for Human Health and Strategies for Containment. Vol. 336, The Resistance Phenomenon in Microbes and Infectious Disease Vectors: Implications for Human Health and Strategies for Containment: Workshop Summary. 2003.
- Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–18.
- Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;(6):25–64.
- Hughes D, Andersson DI. Evolutionary Trajectories to Antibiotic Resistance. Annu Rev Microbiol. 2017;71:579–96.
- Oz T, Guvenek A, Yildiz S, Karaboga E, Tamer YT, Mumcuyan N, et al. Strength of selection pressure is an important parameter contributing to the complexity of antibiotic resistance evolution. Mol Biol Evol. 2014;31(9):2387–401.
- Versporten A, Zarb P, Caniaux I, Gros MF, Drapier N, Miller M, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6(6).
- Salam MA, Al-Amin MY, Salam MT, Pawar JS, Akhter N, Rabaan AA, et al. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Vol. 11, Healthcare (Switzerland). 2023.
- Fadare JO, Ogunleye O, Iliyasu G, Adeoti A, Schellack N, Engler D, et al. Status of antimicrobial stewardship programmes in Nigerian tertiary healthcare facilities: Findings and implications. J Glob Antimicrob Resist. 2019;17.
- Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Vol. 6, Antimicrobial Resistance and Infection Control. 2017.
- Browne AJ, Chipeta MG, Haines-Woodhouse G, Kumaran EPA, Hamadani BHK, Zaraa S, et al. Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet Health. 2021;5(12).
- Ekuma A, Ijezie E, Akpabio T, Onwuezobe I. Survey of antimicrobial consumption in a university teaching hospital in Southern Nigeria. Annals of Tropical Pathology. 2019;10(1).
- Rabie D, Kheder SI. Assessment of Prescribing and Dispensing Practices Based on WHO Core Prescribing Indicators in Hospital and Community Pharmacies in Khartoum State - Sudan. Journal of Medical Informatics and Decision Making. 2020;1(3).
- Amponsah OKO, Nagaraja SB, Ayisi-Boateng NK, Nair D, Muradyan K, Asense PS, et al. High Levels of Outpatient Antibiotic Prescription at a District Hospital in Ghana: Results of a Cross Sectional Study. Int J Environ Res Public Health. 2022;19(16).
- Klein EY, Milkowska-Shibata M, Tseng KK, Sharland M, Gandra S, Pulcini C, et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. Lancet Infect Dis. 2021;21(1).
- Lakoh S, John-Cole V, Luke RDC, Bell N, Russell JBW, Mustapha A, et al. Antibiotic use and consumption in Freetown, Sierra Leone: A baseline report of prescription stewardship in outpatient clinics of three tertiary hospitals. IJID Regions. 2023;7.
- Pauwels I, Versporten A, Drapier N, Vlieghe E, Goossens H. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): Results from a worldwide point prevalence survey in 69 countries. Journal of Antimicrobial Chemotherapy. 2021;76(6).
- Bansal A, Sharma R, Prakash R. Adoption of the World Health Organization access, watch reserve index to evaluate and monitor the use of antibiotics at a tertiary care hospital in India. Perspect Clin Res. 2022;13(2).
- Horumpende PG, Mshana SE, Mouw EF, Mmbaga BT, Chilongola JO, De Mast Q. Point prevalence survey of antimicrobial use in three hospitals in North-Eastern Tanzania. Antimicrob Resist Infect Control. 2020;9(1).
- Mudenda S, Chomba M, Chabalenge B, Hikaambo CN, Banda M, Daka V, et al. Antibiotic Prescribing Patterns in Adult Patients According to the WHO AWaRe Classification: A Multi-Facility Cross-Sectional Study in Primary Healthcare Hospitals in Lusaka, Zambia. Pharmacology & Pharmacy. 2022;13(10).
- Sharland M, Gandra S, Huttner B, Moja L, Pulcini C, Zeng M, et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Vol. 19, The Lancet Infectious Diseases. 2019.
- Mugada V, Mahato V, Andhavaram D, Vajhala SM. Evaluation of prescribing patterns of antibiotics using selected indicators for antimicrobial use in hospitals and the access, watch, reserve (Aware) classification by the world health organization. Turk J Pharm Sci. 2021;18(3).
- 2021 AWaRe classification [Internet]. [cited 2024 Aug 16]. Available from: https://www.who.int/publications/i/item/2021-aware-classification
- Lakoh S, Adekanmbi O, Jiba DF, Deen GF, Gashau W, Sevalie S, et al. Antibiotic use among hospitalized adult patients in a setting with limited laboratory infrastructure in Freetown Sierra Leone, 2017–2018. International Journal of Infectious Diseases. 2020;90.
- Cox JA, Vlieghe E, Mendelson M, Wertheim H, Ndegwa L, Villegas M V., et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Vol. 23, Clinical Microbiology and Infection. 2017.
- Bonniface M, Nambatya W, Rajab K. An evaluation of antibiotic prescribing practices in a rural refugee settlement district in Uganda. Antibiotics. 2021;10(2).
- Maina M, Mwaniki P, Odira E, Kiko N, McKnight J, Schultsz C, et al. Antibiotic use in Kenyan public hospitals: Prevalence, appropriateness and link to guideline availability. International Journal of Infectious Diseases. 2020;99.
- Desalegn AA. Assessment of drug use pattern using WHO prescribing indicators at Hawassa University teaching and referral hospital, south Ethiopia: A cross-sectional study. BMC Health Serv Res. 2013;13(1).
- Kilipamwambu A, Bwire GM, Myemba DT, Njiro BJ, Majigo M V. WHO/INRUD core prescribing indicators and antibiotic utilization patterns among primary health care facilities in Ilala district, Tanzania. JAC Antimicrob Resist. 2021;3(2).
- Lam TP, Chan TH, Sun KS, Lam KF, Kwok KW, Ho PL. Antibiotic prescriptions by medical interns in Hong Kong: influence of the hospital settings and prescription culture. Postgrad Med J. 2021;97(1151).
Medical Journal of Zambia, Vol 51, 4
The Medical Journal of Zambia, ISSN 0047-651X, is published by the Zambia Medical Association.
© This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.