Neuroinflammation in Acute Pseudobulbar Palsy: Key Pathways and Biomarkers
Author title
affiliation goes here
DOI: https://doi.org/10.55320/mjz.51.4.525
Keywords:artery of Percheron, neuroinflammation, pseudobulbar palsy, stroke recovery, thalamic infarction
ABSTRACT
Background: Acute pseudobulbar palsy (PBP) secondary to the artery of Percheron (AOP) infarction presents with sudden dysarthria, dysphagia, and emotional lability, but the role of neuroinflammation in its progression remains unclear.
Purpose: This study aims to clarify how neuroinflammation affects the course and recovery of acute PBP after AOP infarction by identifying key pathways and potential biomarkers for targeted therapies.
Methodology: A literature review from 2018 onwards explored neuroinflammation in stroke and PBP, analyzing data to uncover patterns, gaps, and emerging trends. The Neuroinflammatory Hypothesis of Stroke Recovery guided theoretical analysis.
Results/Conclusion: Neuroinflammation contributes to both initial neural damage and subsequent recovery in acute PBP secondary to AOP infarction. Specific inflammatory markers like cytokines and microglial activity may predict outcomes. Targeted therapies modulating neuroinflammation could improve patient outcomes. This research fills critical gaps in understanding PBP's pathophysiology and informs personalized therapeutic approaches.
INTRODUCTION
Acute pseudobulbar palsy (PBP) is a neurological condition marked by sudden dysarthria, dysphagia, and emotional lability, often resulting from damage to brainstem pathways controlling facial and throat motor functions.[1] While traditionally linked to diffuse brainstem injuries, recent evidence suggests PBP's association with infarctions in the ‘Artery of Percheron’ (AOP), a rare vascular anomaly.[2] AOP infarctions, affecting critical brain regions like the thalami and midbrain, can lead to symmetrical bilateral thalamic and midbrain infarctions, manifesting as acute PBP.[3] Despite its clinical significance, research on the mechanisms and outcomes of PBP secondary to AOP infarction remains limited, particularly concerning neuroinflammatory influences.[4] Neuroinflammation, implicated in stroke outcomes, may significantly affect PBP progression and recovery.[5] Empirical research is urgently needed to elucidate neuroinflammatory pathways in PBP following AOP infarction, potentially yielding biomarkers for prognosis prediction and informing targeted therapies.[6] This study addresses a crucial gap in neurological research by enhancing our understanding of PBP's pathophysiology and offering avenues for improved interventions.
A comprehensive literature study will be conducted to gather and analyze existing research on neuroinflammation in acute PBP secondary to AOP infarction. This method involves systematic searches of scientific databases for relevant articles published from 2018 onwards. Data analysis will focus on extracting and synthesizing information on inflammatory markers, clinical outcomes, and therapeutic interventions. The extracted data will be critically evaluated to identify patterns, gaps, and emerging trends. This analysis will provide a robust foundation for understanding current knowledge, guiding empirical research, and informing the development of targeted therapies to improve patient outcomes.
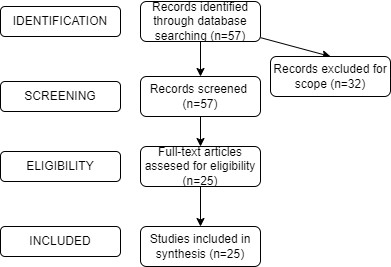
Search Strategies: The search strategy for this study involved systematic searches of scientific databases such as Google Scholar. Keywords and Medical Subject Headings (MeSH) terms related to "acute pseudobulbar palsy," "artery of Percheron infarction," and "neuroinflammation" were used in various combinations. Boolean operators (AND, OR) were employed to refine the search and ensure inclusivity. The search was limited to articles published from 2018 onwards to capture the most recent research in the field.
Inclusion Criteria: Articles were included if they met the following criteria: Articles focusing on acute pseudobulbar palsy secondary to the artery of Percheron infarction and its association with neuroinflammation. Articles published from 2018 onwards to include recent developments. Original research studies, systematic reviews, meta-analyses, and review articles. Articles are written in English to facilitate comprehension and analysis. Exclusion Criteria: Articles were excluded if they met any of the following criteria: Articles not directly related to acute pseudobulbar palsy, artery of Percheron infarction, or neuroinflammation. Articles published before 2018 to focus on recent literature. Study Design: Case reports, letters, editorials, and conference abstracts. Articles not written in English due to language barriers.
Data Synthesis: Data from selected articles were synthesized using a thematic analysis approach. Relevant information, including study objectives, methods, key findings, and conclusions, was extracted and organized systematically. Common themes and patterns related to neuroinflammation in acute pseudobulbar palsy secondary to the artery of Percheron infarction were identified. The synthesized data were then analyzed to identify gaps, emerging trends, and areas requiring further investigation. This process enabled a comprehensive understanding of the current state of research and facilitated the development of the study's conclusions and recommendations.
Literature Review
Neuroinflammation in AOP Infarction
Neuroinflammation is a fundamental component of the pathophysiology of AOP infarction.7 Following the ischemic insult, a cascade of inflammatory responses is triggered, involving various cellular and molecular mechanisms. Among these, microglia, the resident immune cells of the brain, play a central role in orchestrating the neuroinflammatory response.
Upon activation, microglia undergo morphological changes and release pro-inflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α).[8] These cytokines act as signaling molecules, amplifying the inflammatory cascade and recruiting other immune cells to the site of injury. Additionally, microglia release reactive oxygen species and nitric oxide, contributing to oxidative stress and further exacerbating neuronal injury.
While the neuroinflammatory response initially serves a protective function by containing the damage and clearing cellular debris, prolonged or excessive inflammation can have detrimental effects on neuronal survival.[9] The release of pro-inflammatory mediators can disrupt the blood-brain barrier, allowing the infiltration of peripheral immune cells into the brain parenchyma.[10] This infiltration further amplifies the inflammatory response and can lead to secondary neuronal damage and apoptosis.
Importantly, the inflammatory milieu created by neuroinflammation can alter the microenvironment of the brain, influencing neuronal plasticity and repair processes. In the context of AOP infarction, the neuroinflammatory response not only exacerbates acute neuronal injury but also modulates long-term recovery trajectories. Understanding the dynamics of neuroinflammation in AOP infarction is essential for developing targeted therapeutic interventions aimed at mitigating inflammation and promoting neuroprotection.
Neuroinflammatory Response and Clinical Outcomes
Evidence from clinical studies suggests a direct correlation between the extent of neuroinflammatory response and the severity of clinical outcomes in AOP infarction and associated PBP. Several biomarkers of neuroinflammation have been identified, providing insights into the relationship between inflammation and disease progression.
For instance, elevated levels of C-reactive protein (CRP), a well-established inflammatory marker, have been consistently associated with poorer recovery and worse functional outcomes in stroke patients.[10] Studies have demonstrated that higher CRP levels at admission are predictive of increased disability, higher mortality rates, and poorer long-term recovery.11 This association underscores the detrimental impact of systemic inflammation on stroke outcomes and suggests that similar mechanisms may contribute to the clinical trajectory of AOP infarction.
Furthermore, neuroimaging techniques such as positron emission tomography (PET) have been utilized to assess microglial activation, providing valuable insights into the neuroinflammatory response in vivo.[12] Studies have shown that increased microglial activation, as indicated by elevated binding of radioligands targeting microglial cells, correlates with the severity of neurological deficits post-stroke. Specifically, regions of heightened microglial activation coincide with areas of ischemic injury and are associated with worse clinical outcomes.
These findings highlight the importance of neuroinflammation in determining the clinical trajectory of AOP infarction and associated PBP. The correlation between inflammatory markers such as CRP and microglial activation with disease severity underscores the role of neuroinflammation as a key determinant of outcomes in these conditions.[13] Understanding the link between neuroinflammatory responses and clinical outcomes is crucial for identifying potential therapeutic targets and developing strategies to mitigate inflammation and improve patient prognosis.
DISCUSSION
Inflammatory Cytokines as Biomarkers
The identification of biomarkers that can predict outcomes in patients with PBP secondary to AOP infarction holds significant promise for improving prognosis and guiding therapeutic interventions.[14] Recent research has focused on elucidating specific biomarkers associated with neuroinflammation and their potential predictive value in assessing disease severity and recovery trajectories.One group of biomarkers that has garnered considerable attention is inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). Elevated serum levels of these cytokines have been consistently associated with worse functional outcomes in stroke patients. Studies have shown that higher levels of IL-6 and TNF-α at admission correlate with increased disability, greater infarct volume, and poorer long-term recovery.[15] These findings suggest that systemic inflammation, reflected by circulating cytokine levels, may play a significant role in driving the clinical course of AOP infarction and associated PBP.
In addition to inflammatory cytokines, markers of microglial activation have emerged as potential indicators of ongoing neuroinflammation and predictors of recovery. Translocator protein (TSPO), a biomarker expressed by activated microglia, has been investigated as a marker of neuroinflammatory activity in various neurological conditions, including stroke. Studies utilizing PET imaging with TSPO ligands have demonstrated increased TSPO expression in regions of ischemic injury, indicating heightened microglial activation.[16] Importantly, the extent of TSPO expression has been correlated with the severity of neurological deficits post-stroke, suggesting its potential utility as a predictive biomarker for assessing disease severity and monitoring treatment response.
The identification of biomarkers such as inflammatory cytokines and TSPO expression offers valuable insights into the underlying pathophysiology of AOP infarction and associated PBP.[17] These biomarkers not only provide objective measures of neuroinflammatory activity but also hold promise for predicting disease outcomes and guiding therapeutic decision-making. Further research into the predictive value of these biomarkers in larger patient cohorts is warranted to validate their utility in clinical practice and ultimately improve patient care.
The identification of biomarkers associated with neuroinflammation in PBP secondary to AOP infarction not only aids in prognostication but also offers valuable targets for therapeutic intervention. By targeting the underlying inflammatory pathways, it is possible to mitigate the detrimental effects of neuroinflammation and improve recovery outcomes in patients with PBP.
One potential therapeutic approach involves targeting inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). Higher levels of these cytokines are associated with poorer functional outcomes in stroke patients and contribute to neuroinflammation in AOP infarction.[18]
Microglial Activation and TSPO
Another promising therapeutic strategy involves modulating microglial activity, which plays a central role in orchestrating the neuroinflammatory response. Translocator protein (TSPO), a biomarker of microglial activation, has emerged as a potential target for therapeutic intervention. Agents that selectively bind to TSPO receptors, such as benzodiazepines and second-generation TSPO ligands, have been investigated for their neuroprotective effects in various neurological conditions, including stroke. These agents modulate microglial activation and attenuate the inflammatory response, offering a potential avenue for mitigating neuroinflammation in PBP patients. Clinical trials evaluating TSPO ligands in stroke patients have shown promising results in reducing neuroinflammation and improving functional outcomes, suggesting that similar approaches may hold therapeutic promise for patients with PBP secondary to AOP infarction.[19] The identification of biomarkers associated with neuroinflammation in PBP secondary to AOP infarction provides valuable targets for therapeutic intervention. By targeting inflammatory cytokines such as IL-6 and TNF-α or modulating microglial activity through TSPO ligands, it is possible to mitigate the detrimental effects of neuroinflammation and improve recovery outcomes in PBP patients. Further research into these therapeutic strategies is warranted to validate their efficacy and safety in clinical practice and ultimately improve patient care and outcomes.
Integrating clinical data with advanced neuroimaging techniques offers a comprehensive approach to understanding the impact of neuroinflammation on brain structures and function in patients with PBP secondary to AOP infarction. Magnetic resonance imaging (MRI) and positron emission tomography (PET) imaging can provide valuable insights into the structural and functional changes associated with neuroinflammation. MRI allows for high-resolution imaging of the brain, enabling visualization of ischemic lesions in the thalami and midbrain. Lesion mapping using MRI can reveal the extent and distribution of ischemic damage, providing crucial information about the spatial localization of injury. Additionally, diffusion-weighted imaging (DWI) can assess the integrity of white matter tracts and identify regions of ischemic injury not apparent on conventional sequences. Combining structural MRI with functional MRI techniques such as resting-state functional connectivity analysis can further elucidate the functional consequences of ischemic damage on brain networks involved in motor control and sensory processing.
By integrating clinical assessments with advanced neuroimaging techniques, researchers can establish a direct correlation between neuroinflammatory markers, structural damage, and clinical symptoms in PBP secondary to AOP infarction. The severity of ischemic lesions visualized on MRI can be correlated with the intensity of neuroinflammatory responses detected on PET imaging. Furthermore, the spatial distribution of neuroinflammation can be linked to specific clinical manifestations such as dysarthria, dysphagia, and emotional lability.
Overall, integrating clinical and imaging data offers a comprehensive approach to understanding the pathophysiological mechanisms underlying PBP secondary to AOP infarction. This multidimensional approach provides valuable insights into the relationship between neuroinflammation, structural damage, and clinical symptoms, guiding therapeutic decision-making and improving patient outcomes.
By combining clinical assessments with imaging findings, researchers can map the relationship between neuroinflammatory markers and structural damage, providing a holistic view of the disease process in patients with PBP secondary to AOP infarction. This integration enables a comprehensive understanding of the underlying pathophysiology and facilitates the development of precise diagnostic tools and targeted therapies that address both the inflammatory and structural components of PBP.
Clinical assessments, such as neurological examinations and standardized assessment scales, provide valuable information about the severity and nature of neurological deficits in patients with PBP. These assessments allow clinicians to characterize the clinical phenotype of PBP and monitor changes in symptoms over time. Additionally, clinical assessments can identify associated comorbidities and functional impairments that may impact patient outcomes.
Imaging techniques, including MRI and PET imaging, offer complementary insights into the structural and functional alterations associated with neuroinflammation in PBP. MRI provides detailed visualization of ischemic lesions and structural abnormalities in the thalami and midbrain, allowing for precise localization and characterization of the underlying pathology.
Integration of Clinical and Imaging Data
By integrating clinical assessments with imaging findings, researchers can correlate neuroinflammatory markers with specific patterns of structural damage observed on MRI. For example, regions of heightened microglial activation detected on PET imaging may coincide with areas of ischemic injury visualized on DWI or T2-weighted MRI sequences.[20] Furthermore, the severity of neuroinflammatory responses may be correlated with the extent and severity of structural damage, providing insights into the relationship between inflammation and tissue injury in PBP.
This integration of clinical and imaging data facilitates the development of precise diagnostic tools for identifying patients at risk of poor outcomes and guiding therapeutic interventions. By identifying specific neuroinflammatory markers associated with structural damage, clinicians can tailor treatment strategies to target both the inflammatory and structural components of PBP. For example, therapies aimed at reducing neuroinflammation may be combined with interventions targeting neuroprotection and neural repair to optimize patient outcomes.
Overall, the integration of clinical assessments with imaging findings offers a comprehensive approach to understanding the pathophysiology of PBP secondary to AOP infarction. This multidimensional approach provides valuable insights into the relationship between neuroinflammation, structural damage, and clinical symptoms, guiding the development of precise diagnostic tools and targeted therapies for improving patient outcomes.
Therapeutic Implications
The insights gained from understanding the role of neuroinflammation in PBP secondary to AOP infarction have significant therapeutic implications, offering promising avenues for improving patient outcomes. Targeted therapies that modulate neuroinflammatory response hold promise for mitigating neuronal damage and enhancing recovery in patients with PBP.
One potential therapeutic approach involves the use of anti-inflammatory agents to dampen neuroinflammation and reduce neuronal injury. Minocycline, a tetracycline derivative with anti-inflammatory properties, has emerged as a promising candidate for neuroprotection in stroke. Preclinical studies have shown that minocycline inhibits microglial activation and reduces the production of pro-inflammatory cytokines, leading to a reduction in infarct size and improved functional recovery in animal models of stroke.[21] These findings suggest that minocycline may hold therapeutic potential for attenuating neuroinflammation and promoting recovery in patients with PBP secondary to AOP infarction.
In addition to anti-inflammatory agents, other therapeutic strategies aimed at modulating microglial activation and neuroinflammatory pathways may also offer therapeutic benefits in PBP. For example, agents targeting specific signaling pathways involved in microglial activation, such as nuclear factor-kappa B (NF-κB) or mitogen-activated protein kinase (MAPK), could potentially limit neuroinflammation and mitigate neuronal damage. Furthermore, therapies aimed at promoting neuroprotective mechanisms, such as enhancing antioxidant defenses or stimulating neurogenesis, may complement anti-inflammatory strategies and promote tissue repair in the aftermath of AOP infarction.
Importantly, the development of targeted therapies for PBP secondary to AOP infarction requires a comprehensive understanding of the underlying pathophysiological mechanisms, including the temporal dynamics of neuroinflammation and its interactions with other cellular and molecular processes. Clinical trials evaluating the safety and efficacy of these therapeutic interventions in human patients are warranted to validate their utility in clinical practice and ultimately improve patient outcomes. The insights gained from understanding the role of neuroinflammation in PBP secondary to AOP infarction have significant therapeutic implications. Targeted therapies aimed at modulating the neuroinflammatory response hold promise for attenuating neuronal damage and promoting recovery in patients with PBP. Further research into these therapeutic strategies is warranted to translate these findings into clinical practice and improve the management and prognosis of PBP secondary to AOP infarction.
Furthermore, recognizing the dual-phase nature of neuroinflammation in PBP secondary to AOP infarction opens avenues for the development of phase-specific interventions tailored to the evolving pathophysiological processes.
In the acute phase following AOP infarction, therapies could focus on limiting excessive inflammation and neuronal damage. Given that neuroinflammation plays a crucial role in exacerbating ischemic injury during this phase, early interventions targeting inflammatory pathways may mitigate the extent of neuronal damage and improve outcomes. Anti-inflammatory agents such as corticosteroids or non-steroidal anti-inflammatory drugs (NSAIDs) could be administered to dampen the neuroinflammatory response and reduce neuronal apoptosis. Additionally, neuroprotective agents targeting excitotoxicity or oxidative stress may complement anti-inflammatory therapies by preserving neuronal integrity and function.
Conversely, in the chronic phase of neuroinflammation, treatments could aim to enhance reparative processes such as neurogenesis and synaptic plasticity. While neuroinflammation initially serves a protective role in clearing cellular debris and initiating tissue repair, prolonged inflammation can hinder regenerative processes and impede functional recovery. Therapies that promote neuroplasticity and neurorepair mechanisms may therefore be beneficial in the chronic phase of neuroinflammation. For example, growth factors such as brain-derived neurotrophic factor (BDNF) or insulin-like growth factor-1 (IGF-1) could be administered to stimulate neurogenesis and enhance synaptic plasticity, facilitating functional recovery and rehabilitation efforts.[22] Importantly, phase-specific interventions should be tailored to the individual patient's disease course and response to treatment. Serial monitoring of neuroinflammatory markers and imaging findings may help guide therapeutic decision-making and optimize treatment strategies over time. Furthermore, multidisciplinary approaches involving neurologists, neurosurgeons, rehabilitation specialists, and other healthcare providers are essential for coordinating comprehensive care and maximizing patient outcomes. Recognizing the dual-phase nature of neuroinflammation in PBP secondary to AOP infarction offers opportunities for developing phase-specific interventions aimed at targeting the underlying pathophysiological processes. By tailoring treatments to the acute and chronic phases of neuroinflammation, it may be possible to optimize outcomes and improve the management of PBP in patients with AOP infarction. Further research into phase-specific therapeutic strategies is warranted to validate their efficacy and safety in clinical practice and ultimately improve patient care and prognosis.
Longitudinal studies tracking patients from the acute phase through recovery are indispensable for elucidating the temporal dynamics of neuroinflammation and its implications for long-term outcomes in PBP secondary to AOP infarction. These studies provide valuable insights into the evolution of inflammatory responses over time and identify critical windows for therapeutic intervention.
By following patients longitudinally, researchers can capture the dynamic changes in neuroinflammatory markers and imaging findings across different phases of the disease. Serial assessments of inflammatory cytokines, microglial activation, and structural damage allow for a comprehensive characterization of the inflammatory cascade and its impact on neuronal integrity and function. Furthermore, longitudinal studies enable the identification of prognostic biomarkers that predict disease progression and recovery trajectories, facilitating personalized treatment strategies tailored to individual patient needs.
Longitudinal studies also provide an opportunity to evaluate the efficacy of therapeutic interventions at different stages of the disease. By monitoring patients over time, researchers can assess the effects of interventions on neuroinflammatory markers, structural damage, and clinical outcomes, providing valuable data on treatment response and optimization. Additionally, longitudinal studies allow for the identification of factors that influence treatment outcomes, such as patient demographics, comorbidities, and genetic predisposition, informing personalized medicine approaches.
Personalized medicine, guided by the principles of longitudinal studies, aims to tailor treatments to the specific characteristics and needs of individual patients. By integrating clinical, imaging, and molecular data, clinicians can develop personalized treatment plans that optimize efficacy and minimize adverse effects. For example, patients with a more pronounced neuroinflammatory response may benefit from early initiation of anti-inflammatory therapies, while those with impaired neurorepair mechanisms may require interventions aimed at enhancing neuroplasticity and regeneration. Longitudinal studies tracking patients from the acute phase through recovery are essential for understanding the temporal dynamics of neuroinflammation and its impact on long-term outcomes in PBP secondary to AOP infarction. By identifying critical windows for therapeutic intervention and informing personalized treatment strategies, these studies have the potential to improve patient care and prognosis in this debilitating condition. Further research into longitudinal outcomes and personalized medicine approaches is warranted to translate these findings into clinical practice and ultimately improve patient outcomes.
Moreover, the findings from longitudinal studies tracking patients with PBP secondary to AOP infarction can pave the way for personalized medicine approaches.[23] By identifying individual variations in neuroinflammatory responses and their impact on PBP outcomes, clinicians can tailor treatments to the specific needs of each patient. This personalized approach has the potential to optimize recovery and reduce the overall burden of PBP on patients and healthcare systems.
Personalized medicine aims to customize treatment strategies based on the unique characteristics and needs of individual patients.[24] In the context of PBP secondary to AOP infarction, personalized medicine approaches leverage insights from longitudinal studies to develop targeted interventions that address the underlying pathophysiological mechanisms driving neuroinflammation and neuronal injury. High-throughput screening has identified several promising targets for therapeutic intervention in PBP. For instance, recent studies have highlighted the potential of targeting specific inflammasomes (e.g., NLRP3) implicated in the initiation and propagation of neuroinflammation following AOP infarction. Clinical trials exploring inhibitors of these inflammasomes could provide insights into their efficacy in reducing neuroinflammatory responses and improving neurological outcomes.[25]
One aspect of personalized medicine involves identifying prognostic biomarkers that predict disease progression and treatment response. By analyzing longitudinal data on neuroinflammatory markers, imaging findings, and clinical outcomes, clinicians can identify biomarkers associated with favorable or unfavorable prognosis. These biomarkers may include genetic variants, inflammatory cytokine profiles, or imaging markers of neuronal integrity and synaptic function. By stratifying patients based on their biomarker profiles, clinicians can tailor treatment plans to optimize efficacy and minimize adverse effects.
Furthermore, personalized medicine approaches in PBP may involve the development of individualized treatment algorithms that account for patient-specific factors such as age, comorbidities, and treatment preferences. For example, elderly patients with multiple comorbidities may benefit from conservative management strategies aimed at symptom control and rehabilitation, while younger patients with a more robust neuroplasticity may be candidates for more aggressive neuroprotective therapies.
By tailoring treatments to the specific needs of each patient, personalized medicine approaches have the potential to optimize recovery and reduce the overall burden of PBP on patients and healthcare systems. By optimizing treatment efficacy and minimizing adverse effects, personalized medicine approaches can improve patient outcomes and enhance quality of life for individuals affected by PBP secondary to AOP infarction. The findings from longitudinal studies tracking patients with PBP secondary to AOP infarction can inform personalized medicine approaches that optimize treatment strategies based on individual patient characteristics and needs. By tailoring treatments to the specific pathophysiological mechanisms driving neuroinflammation and neuronal injury, personalized medicine approaches have the potential to improve outcomes and reduce the overall burden of PBP on patients and healthcare systems. Further research into prognostic biomarkers and individualized treatment algorithms is warranted to translate these findings into clinical practice and improve patient care.
One limitation of this study is the reliance on preclinical studies and animal models to elucidate the pathophysiological mechanisms underlying PBP secondary to AOP infarction. While these studies provide valuable insights into the underlying biology, there may be differences in disease pathogenesis between animal models and human patients. Future research should focus on validating findings from preclinical studies in human cohorts to ensure the relevance and translatability of the findings to clinical practice.
A promising avenue for future research is the exploration of novel therapeutic targets for neuroinflammation in PBP secondary to AOP infarction. While existing studies have focused on targeting inflammatory cytokines and microglial activation, there may be other pathways involved in neuroinflammation that have yet to be fully elucidated. Future studies should employ high-throughput screening techniques and omics approaches to identify novel targets for therapeutic intervention. Additionally, clinical trials evaluating the efficacy of these novel therapies in human patients are warranted to translate preclinical findings into clinical practice.
CONCLUSION
In conclusion, Acute pseudobulbar palsy (PBP) secondary to artery of Percheron (AOP) infarction involves complex pathophysiological mechanisms, prominently driven by neuroinflammation. This study underscores neuroinflammation's pivotal role in neuronal injury and clinical severity in PBP. Integration of clinical assessments and advanced neuroimaging reveals significant structural and functional changes linked to neuroinflammation, guiding targeted therapies. Longitudinal studies are crucial for understanding neuroinflammation's temporal dynamics and its impact on long-term outcomes. Personalized medicine approaches promise to revolutionize PBP management by optimizing treatment efficacy and improving patient quality of life.
Future Research: Building upon insights gained from mechanistic studies and imaging biomarkers, clinical trials evaluating novel anti-inflammatory agents or immunomodulatory therapies tailored to PBP patients are warranted.
REFERENCES
- Chen Z, He Y, Su Y, Sun Y, Zhang Y, Chen H. Association of inflammatory and platelet volume markers with clinical outcome in patients with anterior circulation ischaemic stroke after endovascular thrombectomy. Neurol Res. 2021;43(6):503-510. doi:10.1080/01616412.2020.1870359.
- Hu D, Ding C, Jiang X, Xiao J, Li C, Zhang L, et al. Elevated levels of inflammation markers predict poor outcomes in acute ischemic stroke patients after intravenous thrombolysis. J Stroke Cerebrovasc Dis. 2021;30(3):105587. doi: 10.1016/j.jstrokecerebrovasdis.2020.105587.
- Hu J, Wang L, Fan K, Ren W, Wang Q, Ruan Y, et al. The association between systemic inflammatory markers and post-stroke depression: a prospective stroke cohort. Clin Interv Aging. 2021; 16:1231-1239. doi:10.2147/CIA.S314131.
- Ouyang J, Xie A, Zhou J, Liu R, Wang L, Liu H, et al. Minimally invasive nanomedicine: nanotechnology in photo-/ultrasound-/radiation-/magnetism-mediated therapy and imaging. Chem Soc Rev. 2022;51(12):4996-5041. doi:10.1039/D1CS01148K.
- Golubnitschaja O, Topolcan O, Kucera R, Costigliola V. 10th Anniversary of the European Association for predictive, preventive and Personalised (3P) Medicine-EPMA World Congress Supplement 2020. EPMA J. 2020;11(Suppl 1):1-133. doi:10.1007/s13167-020-00207-0.
- Ciurea AV, Mohan AG, Covache-Busuioc RA, Costin HP, Glavan LA, Corlatescu AD, et al. Unraveling Molecular and Genetic Insights into Neurodegenerative Diseases: Advances in Understanding Alzheimer's, Parkinson's, and Huntington's Diseases and Amyotrophic Lateral Sclerosis. Int J Mol Sci. 2023;24(13):10809. doi:10.3390/ijms241310809.
- Stuckey SM, Ong LK, Collins-Praino LE, Turner RJ. Neuroinflammation as a key driver of secondary neurodegeneration following stroke? Int J Mol Sci. 2021;22(23):13101. doi:10.3390/ijms222313101.
- Aramideh JA, Vidal-Itriago A, Morsch M, Graeber MB. Cytokine signalling at the microglial penta-partite synapse. Int J Mol Sci. 2021;22(24):13186. doi:10.3390/ijms222413186.
- Kempuraj D, Ahmed ME, Selvakumar GP, Thangavel R, Dhaliwal AS, Dubova I, et al. Brain injury-mediated neuroinflammatory response and Alzheimer's disease. Neuroscientist. 2020;26(2):134-155. doi:10.1177/1073858419848293.
- Yang J, Ran M, Li H, Lin Y, Ma K, Yang Y, et al. New insight into neurological degeneration: Inflammatory cytokines and blood-brain barrier. Front Mol Neurosci. 2022;15:1013933. doi:10.3389/fnmol.2022.1013933.
- Soldozy S, Yağmurlu K, Norat P, Elsarrag M, Costello J, Farzad F, et al. Biomarkers predictive of long-term outcome after ischemic stroke: a meta-analysis. World Neurosurg. 2022;163. doi:10.1016/j.wneu.2021.10.157.
- Brummel NE, Hughes CG, Thompson JL, Jackson JC, Pandharipande P, McNeil JB, et al. Inflammation and coagulation during critical illness and long-term cognitive impairment and disability. Am J Respir Crit Care Med. 2021;203(6):699-706. doi:10.1164/rccm.201912-2449OC.
- Wang Z, Song Y, Bai S, Xiang W, Zhou X, Han L, et al. Imaging of microglia in post-stroke inflammation. Nucl Med Biol. 2023;108336. doi:10.1016/j.nucmedbio.2023.108336.
- Ciongaru DN, Dumitriu AS, Dimitriu BA, Paunica S, Giurgiu MC, Mocanu BF, et al. Correlation between periodontal status and Parkinson's disease; a literature review. J Mind Med Sci. 2024;11(1):24-32. doi:10.22543/2392-7674.1492.
- Gawryś K, Turek-Jakubowska A, Gawryś J, Jakubowski M, Dębski J, Szahidewicz-Krupska E, et al. Platelet-Derived Drug Targets and Biomarkers of Ischemic Stroke First Dynamic Human LC-MS Proteomic Study. J Clin Med. 2022;11(5):1198. doi:10.3390/jcm11051198.
- Guimarães Filho GC, de Oliveira Vitorino PV, Inuzuka S, Barroso AS, Pacífico Alves Filho RP, Melo VA, et al. Pharmacological treatment of hypertension guided by peripheral or central blood pressure: a comparison between the two strategies. Front Cardiovasc Med. 2023;10:1247146. doi:10.3389/fcvm.2023.1247146.
- Werry EL, Bright FM, Kassiou M. TSPO PET Imaging as a Biomarker of Neuroinflammation in Neurodegenerative Disorders. Neurodegener Dis Biomark. 2022;407-427. doi:10.1007/978-1-0716-1712-0_17.
- Meyer JH, Cervenka S, Kim MJ, Kreisl WC, Henter ID, Innis RB. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry. 2020;7(12):1064-1074. doi:10.1016/S2215-0366(20)30255-8.
- Battaglini M, Marino A, Montorsi M, Carmignani A, Ceccarelli MC, Ciofani G. Nanomaterials as Microglia Modulators in the Treatment of Central Nervous System Disorders. Advanced Healthcare Materials. 2024 May;13(12):2304180.
- Kreisl WC, Kim MJ, Coughlin JM, Henter ID, Owen DR, Innis RB. PET imaging of neuroinflammation in neurological disorders. The Lancet Neurology. 2020 Nov 1;19(11):940-50. https://doi.org/10.1016/S1474-4422(20)30346-X.
- Oestreich LK, O'Sullivan MJ. Transdiagnostic in vivo magnetic resonance imaging markers of neuroinflammation. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7(7):638-658. doi:10.1016/j.bpsc.2022.01.003.
- Lu Y, Zhou M, Li Y, Li Y, Hua Y, Fan Y. Minocycline promotes functional recovery in ischemic stroke by modulating microglia polarization through STAT1/STAT6 pathways. Biochem Pharmacol. 2021;186:114464. doi:10.1016/j.bcp.2021.114464.
- Williams HC, Carlson SW, Saatman KE. A role for insulin-like growth factor-1 in hippocampal plasticity following traumatic brain injury. In: Vitamins and Hormones. 1st ed. Vol. 118. Academic Press; 2022:423-455. doi:10.1016/bs.vh.2021.11.009.
- Chougale A, Vedante S, Kulkarni G, Patnawar S. Recent Progress on Biosensors for the Early Detection of Neurological Disorders. Chemistry Select. 2022;7(45). doi:10.1002/slct.202203155.
- Yu Q, Zhao T, Liu M, Cao D, Li J, Li Y, Xia M, Wang X, Zheng T, Liu C, Mu X. Targeting NLRP3 inflammasome in translational treatment of nervous system diseases: an update. Frontiers in pharmacology. 2021 Aug 30;12:707696. https://doi.org/10.3389/fphar.2021.707696
Medical Journal of Zambia, Vol 51, 4
The Medical Journal of Zambia, ISSN 0047-651X, is published by the Zambia Medical Association.
© This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.