Synonymous Myocilin mutations break their silence in a Zambian population attending selected referral eye health facilities
Kangwa I. M. Muma
Department of Surgery, School of Medicine, University of Zambia, Lusaka, Zambia
University Teaching Hospitals – EyeHospital, Lusaka, Zambia
Department of Ophthalmology, School of Medicine and Clinical Sciences, Levy Mwanawasa Medical University, Lusaka, Zambia
Robin Bailey
London School of Hygiene and Tropical Medicine, London, UK
Jessie I. M. Nyalazi
University Teaching Hospitals – EyeHospital, Lusaka, Zambia
George Zulu
University Teaching Hospitals – EyeHospital, Lusaka, Zambia
Tyness S. Mumba-Malisawa
University Teaching Hospitals – EyeHospital, Lusaka, Zambia
Lillian M.L. Chinama-Musonda
Eye Department, Levy Mwanawasa University Teaching Hospital, Lusaka, Zambia
Kachikonyo Sibande-Muma
University Teaching Hospitals – EyeHospital, Lusaka, Zambia
Patrick Kaonga
Tropical Gastroenterology and Nutrition Group (TROPGAN), Internal Medicine Department, University Teaching Hospitals – Adult Hospital, Lusaka, Zambia
Edgar Simulundu
Department of Disease Control, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia
DOI: https://doi.org/10.55320/mjz.49.3.370
Keywords:Myocilin (MYOC), Mutation, Primary Open Angle Glaucoma, Synonymous
ABSTRACT
Objective: Primary open-angle glaucoma in Zambia has an earlier age of onset and appears to be more clinically severe. Myocilin mutations are associated with primary open angle glaucoma in multiple populations. Therefore, we investigated the role of myocilin gene mutations in Primary Open Angle Glaucoma in a Zambian population.
Methods: The unrelated primary open-angle glaucoma patients and unaffected controls recruited were from the University Teaching Hospitals Eye Hospital, Kitwe Teaching Eye Hospital and Lusaka Adventist Eye Hospital. A complete eye examination, including visual field assessment, was performed in all cases and controls.Genomic DNA was extracted from whole peripheral blood, then subjected to polymerase chain reaction to amplify exons, flanking introns and promoter regions of the myocilingene. The amplified products were screened for base mutations by auto sequence based on the Sanger method. The analyses of findings included odds ratios, chi-square, bivariate, multivariate and conditional logistic regression with 95% confidence interval and at a 5% level of significance.There was also a comparison between the identified mutations and the previously reported myocilinmutations.
Results: Unrelated 165 Primary Open-Angle Glaucoma patients and unaffected 173 controls enrolled for the study. The analysis revealed 4 variants of MYOC mutations in 49 participants of the 338. The mutations included one synonymous (silent) mutation (Thr474Thr; 45/338) and three missense mutations (Ala446Thr; 16/338), (Leu158Arg; 4/338) and (Arg342Lys; 1/338). The study observed two previously reported mutations, Ala446Thr and Arg342Lys, as glaucoma causing mutations. The missense mutation, Ala446Thr, was found in 16 participants who also had silent mutations broken down as eight cases and eight controls. One control had two variants, Ala446Thr and Arg342Lys. Twenty (20) controls and 25 cases had the synonymous or silent (neutral) mutation, (Thr474Thr). The occurrence of Thr474Thr and Ala446Thr in the same cases and controls was compelling evidence to think that the synonymous mutations were not as silent as previously reported. The Thr474Thr and Ala446Thr mutations exhibited identical primary open-angle glaucomaphenotypic features in both cases and controls. In the remaining 17 cases and 12 controls the phenotype were still the same. The clinical profile in Thr474Thr mutation was statistically significant for female gender, younger age group (<40 years), older age group (≥ 65 years), positive family history, poor visual acuity, severe visual field changes, diffuse retinal nerve fibre layer defects, peripapillary atrophy surrounding the optic nerve head and high cup disc ratio between 0.8 and 1.0.
Conclusions: The silent myocilin mutations, Thr474Thr, break their silence in a Zambian population. The silent mutation Thr474Thr found was associated with primary open-angle glaucoma phenotype and appeared to be a glaucoma-causing.
INTRODUCTION
Glaucoma is a group of non-communicable heterogeneous disorders that cause progressive apoptosis of retinal ganglion cells leading to optic nerve degeneration, excavation and corresponding visual field defects (VFD).[1, 2] Glaucoma is asymptomatic and as such, most of the time, the patients are unaware of the disease.[2] It is usually discovered during evaluation for other eye conditions or when the disease is advanced.[3, 4] The disease eventually leads to visual impairment and blindness.
Glaucoma may be classified based on aetiology as primary or secondary, based on the age of onset as congenital, infantile, juvenile or adult, and based on the anterior chamber angle as Primary Open Angle Glaucoma (POAG) or Primary Congenital Glaucoma (PCG) or Primary Angle Closure Glaucoma (PACG).[5] Juvenile-Onset Open-Angle Glaucoma(JOAG) is said to be a rare subset of POAG.[5] The risk factors for POAG include age, sex, black race, diseases like hypertension (HTN) and diabetes, lifestyle habits such as smoking and alcohol consumption.[6] Other risk factors are exfoliation syndrome, myopia, positive family history of glaucoma and decreased perfusion pressure.6Genetic mutations and central corneal thickness are other risk factors for POAG still being explored.[7, 8] A study conducted at the University Teaching Hospitals – Eye Hospital, Kitwe Teaching Eye hospital and Lusaka Adventist Eye Hospital found the prevalence of myocilin POAG gene mutation at 14.5%.[9] This was much higher than what was reported by other studies and certainly showed that genetic mutations were a risk for glaucoma in the Zambia population.[9]
A study conducted in Zambia at the University Teaching Hospital (UTH) Eye Department in 2013, the prevalence of POAG was remarkably higher (19.0%) than what was reported in a study involving African-derived persons in East Baltimore, Barbados and other African indigenous surveys in West, East and Southern Africa.[7] The high prevalence in this study was attributed to the inclusion of participants aged 20 to 39 years in the studyand also due to the fact that this was a Hospital based study. Similarly, in a study conducted at the UTH, JOAG prevalence was found to be 8.6% in the black Zambian population, suggesting that JOAG was a common condition in the black Zambian people.[10] These findings gave an insight on how the glaucoma problem was in the Zambian setting.
Genetic risk factors are known to contribute to POAG, although it has a complex inheritance pattern that confounds many approaches used to study Mendelian traits.[10]
Fifteen POAG-associated loci and over seventy unique mutations have been identified with many of these mutations being specific to a single population or ethnic group.[11-15] Within the loci several candidate genes have been identified including myocilin (MYOC)[16] , optineurin[17] , WD repeat-containing protein 36 (WDR36)[18] , and cytochrome p450 1B119. Of all these mutations, MYOC (MYOC; accession identifier NM_000261) has been found to harbour more glaucoma-causing mutations than any other identified risk gene[12, 20] , with over eighty mutations identified in different populations.[21, 22]
Synonymous mutations (also known as silent mutations) refers to the substitution of one base for another in an exon of a gene coding for a protein.[23] This mutation is possible because some amino acids are coded for by more than one three base pair codons.[23] Synonymous mutations are said to be silent mutations, although the mutations are not always silent.[23, 24] They are also said to be a specific type of neutral mutation. [24] As such silent mutations in DNA or RNA are thought not to have an observable effect on the organism's phenotype. Synonymous mutations can affect transcription, splicing, mRNA transport and translation any of which could alter the functionality of the tissue or organ and eventually the phenotype, rendering the synonymous mutation non-silent.[25] Research further suggests that alterations in silent mutations to the triplet code can affect protein translation, efficiency, folding and function.[26, 27] As the structure of proteins determines its function; a protein must be able to fold correctly into its tertiary form so that it can function properly. In silent mutations, the folding and functioning of the proteinare disturbed.[23, 26] Kimchi-Sarfaty etal. showed that proteins containing at least one of the silent mutations had a subtly different shape compared with normal proteins which turned out to replace common triplets with much rarer ones.[24] They concluded that it was evident that proteins made of identical amino acids behaved differently and had significant clinical phenotypic manifestations.[24]
Pearson Hellen observed that silent mutations changed the way RNAbridged with DNA to protein production.[28] Pagani et al., concluded that even the most benign-looking polymorphism in an exon should not be ignored as it may affect the splicing processthat inactivate the protein to prevent cystic fibrosis.[29] Komarfurther reported that silent mutations with important functions could be scattered across the human genome.[27] He encouraged researchers to re- examine mutations in numerous genes brushed aside previously. He concluded that understanding these silent mutations could one day help doctors to personalize medicines to match a patient's genetic profile.[27]
This study attempted to establish the contribution of MYOC mutations to POAG in the Zambian population. It also tried to identify novel mutations for future functional work and genetic screening. Therefore, we investigated the role of myocilin mutations in POAG in a Zambian population.
METHODS
This was a case-control study. The cases and controls were matched for age, gender and ethnicity. The age difference was ± two years old, because this difference could not have ocular anatomical differences between the matched participants. For the purposes of the study, ethnicity was categorised based on the officially recognised seven national language groupings of Tonga, Nyanja, Lozi, Kaonde, Luvale, Lunda and Bemba. For each participant, this was determined by the language their mother spoke.
The participants recruited were from University Teaching Hospitals Eye Hospital(UTHs – EH), Kitwe Teaching Eye Hospital (KTEH) and Lusaka Adventist Eye Hospital (LAEH) for a total of 165 cases and 173 controls. All participants consented to study participation in writing. Subjects with POAG were unrelated and met the inclusion criteria of: 1) age of equal to or greater than 18 years; 2) glaucomatous optic neuropathy in at least one eye; and 3) Visual Field (VF) loss consistent with optic nerve damage in at least one eye. Glaucomatous optic neuropathy was defined as a cup-to-disc ratio greater than 0.7 or focal loss of the nerve fibre layer resulting in a notch, associated with a glaucomatous Visual Filed Defect (VFD). Visual Fields were performed using standard Humphrey's Visual Field (HVF) perimetry. The anterior chamber angle in all the POAG cases was measured using gonioscopy. Intraocular Pressure (IOP) was measured by applanation tonometry. The exclusion criteria for POAG subjects included the diagnosis of a secondary form of glaucoma or a history of ocular trauma. The normal controls were recruited specifically for this study and the visual field was part of the eye examination.
The control subjects were unrelated and met the criteria of: 1) no first degree relative with glaucoma; 2) IOP less than 21 mmHg in both eyes without treatment; 3) no evidence of glaucomatous optic neuropathy in either eye and 4) normal VF in both eyes.
All the participants presented as outpatients and underwent a thorough examination by an experienced ophthalmologist.
This study adhered to the tenets of the Declaration of Helsinki. The University of Zambia Biomedical and Research Ethics Committee reviewed and granted authority to conduct the research. The three Hospital sites also allowed the research to go on. After the clinical examination, DNA samples were collected from 5 ml peripheral blood acquired from each of the 338 participants and extracted using the QIAGEN MINI COLUMN Kit per the manufacturer's instructions. DNA was kept in at least 5 aliquots and stored at – 80oC for sequencing later. DNA concentrations were measured using a NanoDrop 1000 spectrophotometer and normalised to 10 ng/ml. The DNA was extracted at the University Teaching Hospitals Tropgan Laboratory and dispatched on ice to the University of Zambia, School of Veterinary Medicine, Department of Disease Control Virology Molecular Laboratory within Lusaka for MYOC gene screening. The gel electrophoresis and spectrophotometry determined the quality and quantity of purified genomic DNA, respectively. Primers flanking the entire coding sequence of MYOCwere procured from the Commercial Laboratory in the United Kingdom. Table 1 below provides a list of the primers that were used in the study to determine the role of POAG genes in the Zambian population.
Table 1: List of PCR Primers for MYOC (Myocilin) Exon Sequencing in a Zambian population
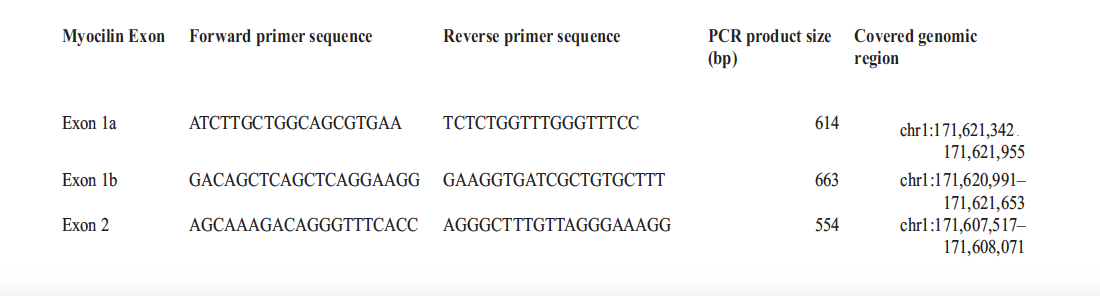
The targeted region covered at least 80 base pairs into each intron to screen for potential mutations affecting exon splicing. Dream Taq DNA polymerase was used for all the polymerase-chain reactions (PCR). The PCR amplifications were performed in Applied Biosystems Veriti 96 Well Thermal Cycler by Life Technologies Model number 9902 made in Singapore in 2015. Completed PCR reactions were purified and sequenced using the Sanger method. Sequencing was performed in a Hitachi Applied Biosystems 3500 genetic analyser model 622-0010 of 2016. The raw sequence data obtained was edited using the Genetyx version 12 software package. After that, blasting and genetic analysis and were performed based on MYOC sequence (GenBank NM_000261) from the NCBI. Sequence alignment of the MYOC wild type and mutant sequences was done using Clustal Omega. The Expasy Bioinformatics Resource Portal was used to translate the mutant MYOC to the protein sequence.
Chi-square test was applied to test the association of MYOC alleles and other variables namely sex, age group, MYOC mutations, family history of glaucoma andethnicity. Odds ratio (OR) had corresponding 95% confidence intervals and p- values were applied to measure association with age, sex, CCT, MYOC mutations, positive family history (exposure) and POAG (outcome). The OR was used to compare the odds of genetic mutation expression and having POAG. The disease severity at presentation using a worse eye VFD and with binocular field defects at presentation was analysed separately using chi square or Pearson's correlation. The case-control confounders were ruled out using the conditional bivariate. Multiple conditional logistic regressions was applied to test the association between the presence of POAG and the variables (genetic mutation, age, sex, history of glaucoma).
RESULTS
A total of 165 cases and 173 controls participated in the study. The cases and controls were matched for age, sex and ethnicity. The male participants made up 51.2% and females 48.8%. The Bemba speaking people were the majority (48.2%), followed by the Nyanja speaking (21.3%), then Tonga speaking (13.6%) and the least were the Lunda speaking (2.9%). In terms of age group, the participants aged below 40 years of age made up 31.4% and these above 65 years represented 26.6%. Table 2 below shows participants' demographics.
Table 2: Socio-demographic characteristics of the case-control study participants at the UTHs EH, KTEH, and LEH, n = 338
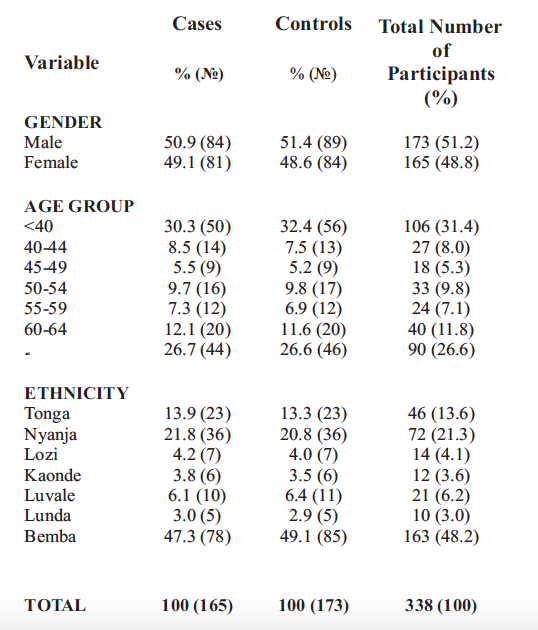
The POAG cases had a mean age of 51.3±?17.9 years, while the mean age for the controls was 55.3±15.8 years. Among the study cases, 84 (50.9%) were males and 81 (49.1%) were females (Table 2). There was no statistical difference between the two sexes. Among the study controls, 84 (48.6%) were males and 89 (51.4%) were females (Table 2).
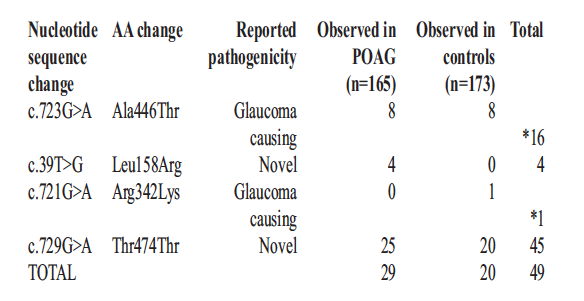
The analysis revealed 4 polymorphisms of MYOC mutations in 49 participants of the 338. The mutations included one silent mutation (Thr474Thr; 45/338) and three missense mutations (Ala446Thr; 16/338), (Leu158Arg; 4/338) and (Arg342Lys; 1/338). All the 16 participants with the missense mutation Ala446Thr (16/338), were part of the 45 who had a silent mutation. The codon usage in the mutation changed to codons code for different amino acids, as shown in Table 3 below.
Table 3: List of Polymorphisms, Original Base, Mutation Base, Original and Mutated Codon as well as Original and Mutated Amino Acids

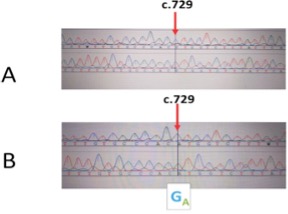
Figures 1 below shows the picture result of the MYOC silent mutationThr474Thr.
All these changes, c.723G>A (Ala446Thr), c.39T>G (Leu158Arg), c.721G>A (Arg342Lys)andc.729G>A (Thr474Thr) appeared to be associated with glaucoma. Ala446Thr was observed in eight controls and eight cases, whereas Leu158Arg was observed in four cases and not in any of the control participants. Both Arg342Lys and Ala446Thr were observed in one control only. Leu158Arg seemed to have been a novel missense mutation. Leu158Arg and Ala446Thr MYOC mutations appeared to cause a clinically distinguishable form of glaucoma when compared to cases that did not carry mutations in MYOC.
The study identified, Thr474Thr, silent variant in 20 controls and 25 cases (Table 4). There was no statistical difference between the cases and controls when it came to the presence of Thr474Thr; p=0.342. Arg342Lys variant was found in no cases, but in one control. The variant c.723G>A was equally distributed among the controls, eight (50%) and the cases, eight (50%). Sixteen participants were found to carry multiple non-synonymous variants. The 16 individuals carried Ala446Thr and Thr474Thr and one of the controls also Arg342Lys to make it three variants; Thr474Thr,Arg342Lys and Ala446Thr. In all, 7.7% (29/338) of the cases carried missense mutations (Leu158Arg, Arg342Lys and Ala446Thr).All these findings are illustrated in Table 4 below.
Table 4: List of Coding Variants and Amino Acid Change Identified from MYOC Exon Sequencing in the Zambian Population, n = 49
When conditional logistic regression (conditioned on age, sex and ethnicity), was conducted CCT, IOP and family history were associated with POAG. The glaucoma cases with a positive history of glaucoma in the family were 108 times more likely to develop glaucoma, and this was statistically significant (p = 0.008). The cases also had 1.697 chances of having raised intraocular pressure and this was equally statistically significant (0.002).
There was no statistical association between MYOC mutations and POAG (p=0.921, p=0.700 and p=0.992). All these findings are illustrated in Table 5 below.
Table 5: Conditional Logistic Regression to Determine Factors Associated with POAG in Cases and Controls (n = 338)
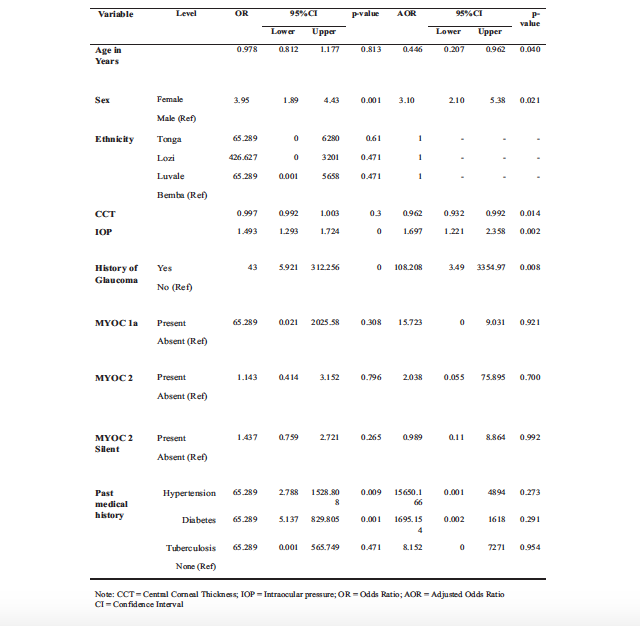
Table 6 Association of Family History of Glaucoma and the Myocilin Mutations (n = 49)
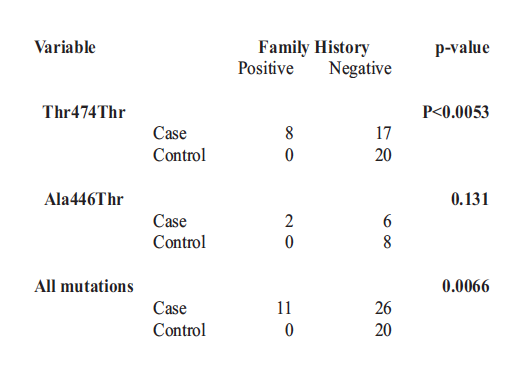
The odds of having a positive family history with MYOC mutations were 7.78 (CI, 4.44,20.56). There was a very significant association between Thr474Thr and Ala446Thr, p<0.001. There was equally a very significant association between MYOC mutations and positive family history of glaucoma in both bivariate and multivariate analysis, p=0.001 and p<0.001
DISCUSSION
Myocilin exists in the form of MYCO protein found in multiple ocular tissues. However, its interaction with the mitochondria in the trabecular meshwork (TM) and astrocytes appear to be specific. Despite extensive research, it remains unclear how myocilin mutations lead to glaucoma.[30, 31] He et al., reported that TM cells overexpressing Pro370Leu mutant MYCO demonstrate features of mitochondrial dysfunction and, thus, Pro370Leu mutant MYCO may increase the vulnerability of TM cells to cellular injury and cause impaired function and even cell death.[32] Cell death is what leads to glaucoma. The MYCO further causes deregulation of calcium channels causing mitochondrial membrane depolarisation in TM cells, TM contraction, and subsequently leading to reduced outflow and IOP elevation.[32] This study did not assess TM genetic studies which would be essential to do so in future.
Research suggests that genetic alterations to the triplet code that do affect protein translation, efficiency, folding and function are referred to as silent mutations or synonymous mutations.[23, 26] These mutations in DNA or RNA are thought mainly not to have any observable effect on the organism's phenotype.[26, 27] They are said to be a specific type of neutral mutation. Several studies have reported that the so-called synonymous mutations are not always silent, nor vice versa.[23-25]
Synonymous mutations can affect transcription, splicing, mRNA transport and translation, any of which could alter phenotype, rendering the synonymous mutation non-silent.[26, 27] As the structure of proteins determines its function, a protein must be folded correctly into its tertiary form so that the protein would function properly. In silent mutations, the folding and functioning of the protein are disturbed.[23-25] Proteins containing at least one of the silent mutations has a different shape compared with normal proteins.[24] Pearsonreported thata team led by Francisco Baralle at the International Centre for Genetic Engineering and Biotechnology in Trieste, Italy, demonstrated that silent mutations gene were responsible for the lung disease called cystic fibrosis.[28] The disease was caused by splicing changes that inactivate the protein to prevent cystic fibrosis. Similarly, silent mutations could be responsible for such conditions as glaucoma because of the altered protein function. If MYOC was protective initially, once it became mutated, it could predispose one to glaucoma.
In this study, the codon usage in the silent mutation (Thr474Thr) changed from ACG to ACA and both codons code for threonine. The guanine base was replaced with the adenine base which is also a purine. Guanine is more complicated than adenine, implying that the protein could have become unstable by introducing an unstable adenine. Although adenine and guanine are both purines, their chemical structures are fundamentally different. Adenine contains an amine group on C-6 and an additional double bond between N-1 and C-6 in its pyrimidine ring. Guanine has an amine group on C-2 and a carbonyl group on C-6 in its pyrimidine ring. These striking differences between guanine and adenine can undoubtedly lead to severe structural and functional changes of the proteins in MYCO hence the mutation.[33]
The above explanation makes logical sense to postulate that the silent mutations could have influenced not only the protein structure but the functionality too considering that adenine and guanine have different properties which would make the protein behave differently. The complementary pairs formed by adenine and guanine is another difference that can cause mutation in MYOC. Whereas adenine forms complementary base pairs with thymine in DNA and uracil in RNA, guanine forms complementary base pairsin both DNA and RNA are formed with cytosine. The molecular mass of adenine is 135.13 g/mol, while for guanine is 151.13 g/mol. The difference in molecular weight of 16 g/mol between the two purines is very significant at molecular level and it can cause structural change in the proteins formed from the two.
Guanine would form a more substantial and stable protein than adenine as it is a heavier base. The solubility of adenine in water is 0.103 g/100 mL whereas Guanine is insoluble in water. Protein containing adenine would dissolve easily in water, making the protein even more unstable. This solubility is another fundamental difference that can lead to alteration in protein structure and function. Adenine and guanine differ from their functional groups, which are attached to the purine core of each molecule.[33]
Due to the preceding possible explanation, the altered protein content would not be able to fold correctly into its tertiary form so that it could function adequately.[23, 27] The disturbance in the functionality of the protein could lead to many medical conditions such as glaucoma. In this study, there are other compelling reasons to think that the synonymous polymorphism may not be silent as to such. The very high prevalence (19.0%) of POAG, strong positive family history and high prevalence of Juvenile onset Open Angle Glaucoma (JOAG)(8.6%) are among the compelling reasons to convincingly state that the synonymous mutations could be the fundamental basis behind all this huge glaucoma burden.[7, 10] A positive family of glaucoma was strongly associated with both the development of glaucoma and presence MYOC mutations. All MYOC mutations in this study led to the development of POAG contrary to other reports that synonymous mutations were silent.[34, 35] In addition all the cases that had the silent mutation, Thr474Thr, had severe form of POAG clinically and their VA was <6/60. The clinical profile of Thr474Thr was statistically significant for female gender, younger age group (<40 years), older age group (≥65 years), positive family history, poor VA, severe visual field changes, diffuse retinal nerve fibre layer defects and peripapillary atrophy surrounding the optic nerve head. The cup disc ratio was high (between 0.9 and 1.0) in all of them, had severe visual field defects affecting the area within 10° from fixation and their IOP was more than 35mmHg and all these findings were bilaterally in 96% of the cases with Thr474Thr mutation. The cases with Thr474Thr mutation had severe, bilateral disease and all of them had a positive family history of glaucoma. They also had presented with advanced disease to the Hospital. The younger cases with Thr474Thr mutation had high IOP and severe glaucomatous damage at presentation compared to the older cases.
These cases presented with eye pain as one of the reasons for coming to the Hospital and had a short history of symptoms.The aforementioned clinical profile describes a severe phenotypic presentation of Thr474Thr mutation associated with visual impairment and blindness.When it came to the controls who had Thr474Thr mutation, they had a positive family history for POAG(for second degree relatives)and their IOP was on the higher side of normal range which was from 19 mmHg to 21mmHg. These phenotypic findings could not have been coincidental, but fundamental ones especially that they were peculiar to the cases and controls with the Thr474Thr mutation and strongly supported the POAG clinical profile. The association between family history of POAG and Thr474Thr mutation was extremely significant, p<0.001, and both groups had over 108 odds of having POAG. These findings were a clear demonstration of the silent MYOC mutations defying their silent nature and revealing their dominant role in the causation of POAG.
The Arg342Lys mutation in one control exhibited the same phenotypic features as with those with mutation Thr474Thr. Similarly, the four cases with the mutation Leu158Arg exhibited the same phenotypic features as the cases which had Thr474Thr. Ideally patients with the mutation Thr474Thr should have exhibited very mild clinical features of POAG and the controls should not have exhibited any clinical features suggestive of POAG if it was truly silent.
CONCLUSION
This finding of the study is a clear manifestation of the mutation Thr474Thr not being silent or neutral polymorphism in nature. The silent mutation Thr474Thr found appears to be glaucoma-causing. This study has helped to establish the contribution of MYOC mutations to POAG in the Zambian population, which is a sub-Saharan population. It can also serve as a basis upon which future works can be developed in POAG genetic screening in Zambia and identify novel mutations.
ACKNOWLEDGEMENTS
The authors wish to thank the Tropical Gastroenterology and Nutrition Group (TROPGAN) team led by Prof. Paul Kelly for allowing us to use the laboratory facilities. The entire TROPGAN team (Prof. Kelly, Dr. Mpala Mwanza-Lisulo, Dr. Caroline Chisenga, Ms. Ellen Besa and Ms. Kanekwa Zyambo) made us feel at home in their laboratory. We further wish to thank the School of Veterinary Medicine team led by Dr. Muso Munyeme for allowing us to use the lab facilities. The entire team (Dr. Musso Munyeme, Mr. Joseph Ndebe, Mr. Penjani Kapila, Mr. Propher Chimpala and Mr. Joseph Mwanza) made us complete the study. We will be failing in our duties if we did not acknowledge the support we received from the Ministry of Health (MoH) headquarters at Ndeke House.
MoH granted us the authority to conduct the study at the University Teaching Hospitals – Eye Hospital and Kitwe Teaching Eye Hospital. We also appreciate the Lusaka Adventist Eye Hospital for allowing us to conduct the study at their facility.
REFERENCES
- Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol 1996; 80:389-93.[PMID: 8695555]
- Weinreb RN, Khaw PT (2004) Primary open- angle glaucoma. Lancet 363(9422): 1711-1720.
- Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD et al. (1991) Relationship between intraocular pressure and primary open-angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol 109(8): 1090-1095.
- Klein BE, Klein R, Sponsel WE, Franke T, Cantor LB et al. (1992) Prevalence of glaucoma.The Beaver Dam Eye Study. Ophthalmology 99(10): 1499-1504.
- Faiq, M., Sharma, R., Dada, R., Mohanty, K., Saluja, D., Dada, T., 2013. Genetic, Biochemical and Clinical Insights into Primary Congenital Glaucoma. J Curr Glaucoma Pract. 7(2):66-84. doi: 10.5005/jp-journals-10008- 1140. Epub 2013 May 9. PMID: 26997785; PMCID: PMC4741182.
- Weih, L.M., Nanjan, M., McCarty, C.A., and Taylor, H.R., 2001. Prevalence and predictors of open-angle glaucoma: results from the visual impairment project. Ophthalmology. 108:1966- 1972. Full Text | Web of Science | PubMed
- Muma MKI., Bailey R. and Michelo C., Primary Open Angle Glaucoma as seen at the University Teaching Hospital, Lusaka, Zambia, Journal of Ophthalmology of Eastern Central and Southern Africa, 2019 Vol 22 ¹ 2, p 43 – 47.
- Muma MKI, Nyalazi JIM, Mumba T, Zulu G, Syakantu G, Chinama – Musonda LM, Bailey R, Simulundu E, Michelo C, Central corneal thickness and its relationship with IOP, visual fields and optic disc parameters among glaucoma patients attending Eye Hospital at University Teaching Hospital in Lusaka, Zambia, 2019, Journal of Ophthalmology of Eastern Central and Southern Africa, Vol 23 ¹ 1; P20-27.
- Muma I. M. Kangwa, Bailey Robin, Nyalazi I. M. Jessie, Zulu George, Malisawa-Mumba S. Tyness, Musonda - Chinama M. L. Lillian, Muma-Sibande Kachikonyo, Kaonga Patrick & Simulundu Edgar (2022). Myocilin Mutations in a Zambian Population Attending Selected Referral Eye Health Facilities. Glob Acad J Med Sci; Vol-4, Iss-2 pp- 45-55.
- Muma, MKI., Mboni, C., Mwale, C., Sibande - Muma, K., Nyalazi, J., Zulu, G., Mumba - Malisawa, T., Zulu, R., Mulenga, P., Chipalo - Mutati, G., Musonda, L., Kaonga, P., Simulundu, E., & Michelo, C. (2020). Juvenile- onset Open-Angle Glaucoma at the University Teaching Hospitals - Eye Hospital, Lusaka Zambia. Medical Journal of Zambia, 47(2), 112 - 124. Retrieved from https://mjz.co.zm/ index.phpmjz/article/view/692
- Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Exp Eye Res 2009; 88:837-44. [PMID: 19061886]
- Hewitt AW, Mackey DA, Craig JE. Myocilin allele-specific glaucoma phenotype database. Hum Mutat 2008a; 29:207-11. [PMID: 17966125]
- Gong G, Kosoko-Lasaki O, Haynatzki GR, Wilson MR. Genetic dissection of myocilin glaucoma. Hum Mol Genet 2004; 13:R91-102. [PMID: 14764620] 1
- Thylefors B., Negrel A. D., Pararajasegaram R., and Dadzie K. Y., 1995, Global data on blindness. Bull World Health Organ, 73:115- 121. Web of Science | Pubmed 1
- Leske M. C., Connell A. M., Schachat A. P., and Hyman L., 1994, The Barbados Eye Study. Prevalence of open-angle glaucoma. Arch Ophthalmol 112(6): 821-829.
- Wiggs J. L., Damji K. F., Haines J. L., Pericak- Vance M. A., and Allingham R. R., 1996, The distinction between juvenile and adult-onset primary open-angle glaucoma. Am J Hum Genet58 (1): 243-244.
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open-angle glaucoma. Science 1997; 275:668- 70. [PMID: 9005853]
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Héon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 2002; 295:1077-9. [PMID: 11834836]
- Monemi S, Spaeth G, DaSilva A, Popinchalk S, Ilitchev E, Liebmann J, Ritch R, Héon E, Crick RP, Child A, Sarfarazi M. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum Mol Genet 2005; 14:725-33. [PMID: 15677485]
- Fan BJ, Wang DY, Lam DS, Pang CP (2006a) Gene mapping for primary open-angle glaucoma. Clin Biochem 39(3): 249-258.
- Hewitt AW, Sharma S, Burdon KP, Wang JJ, Baird PN e t a l . ( 2008 b) Ancestral LOXL 1 variants are associated with pseudoexfoliation in Caucasian Australians but with markedly lower penetrance than in Nordic people. Hum Mol Genet 17(5): 710-716.
- Fingert JH, Stone EM, Sheffield VC, Alward WL (2002) Myocilin glaucoma. Surv Ophthalmol 47(6): 547-561.
- Zhou T, Ko EA, Gu W, Lim I, Bang H, Ko JH (31 October 2012). " Non- silent story on synonymous sites in voltage-gated ion channel genes". PLOS One . 7 ( 10 ): e 48541 . DOI:10.1371/journal.pone.0048541. PMC 3485311. PMID 23119053.#
- Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM (January 2007). "A "silent" polymorphism in the MDR1 gene changes substrate specificity". Science. 315, (5811): 525–8. DOI:10.1126/ science.1135308. PMID 17185560.
- Goymer P (February 2007). "Synonymous mutations break their silence". Nature Reviews Genetics. 8 (2): 92. DOI:10.1038/nrg2056.
- Czech A, Fedyunin I, Zhang G, Ignatova Z (October 2010). "Silent mutations insight: co- variations in tRNA abundance as a key to unravel consequences of silent mutations". Molecular BioSystems. 6 (10): 1767–72. DOI:10.1039/c004796c. PMID 20617253.
- Komar AA (August 2007). "Silent SNPs: impact on gene function and phenotype". Pharmacogenomics . 8 ( 8 ): 1075 – 80 . DOI:10.2217/14622416.8.8.1075. PMID 17716239
- Pearson Helen, Silent mutations speak up, Published online 21 December 2006 | Nature | DOI:10.1038/news061218-12
- Pagani F, Stuani C, Tzetis M, Kanavakis E, Efthymiadou A, Doudounakis S, Casals T, Baralle FE., New type of disease-causing mutations: the example of the composite exonic regulatory elements of splicing in CFTR exon 12. Hum Mol Genet. 2003 May 15;12(10):1111-20. PMID: 12719375
- Liu Y, Allingham RR. Molecular genetics in glaucoma. Exp Eye Res 2011; 93:331-9. [PMID: 21871452]
- Fingert JH, Robin AL, Stone JL, Roos BR, Davis LK, Scheetz TE, Bennett SR, Wassink TH, Kwon YH, Alward WL, Mullins RF, Sheffield VC, Stone EM. Copy number variations on chromosome 12q14 in patients with normal-tension glaucoma. Hum Mol Genet 2011; 20:2482-94. [PMID: 21447600]
- He Y., Leung K. W., Zhuo Y. H., and Ge J., Pro370 Leu mutant myocilin impairs mitochondria functions in human trabecular meshwork cells. Mol Vis 2009; 15: 815-825
- Panawala L, 2017, Difference Between Adenine and Guanine, https://www.researchgate.net/ publication/316984935
- Melki R, Idhajji A, Driouiche S, Hassani M, Boukabboucha A, Akhayat O, Garchon H, Belmouden A. Mutational analysis of the Myocilin gene in patients with primary open- angle glaucoma in Morocco. Ophthalmic Genet 2003; 24:153-60. [PMID: 12868033]
- Challa P, Herndon LW, Hauser MA, Broomer BW, Pericak-Vance MA, Ababio-Danso B, Allingham RR. Prevalence of myocilin mutations in adults with primary open-angle glaucoma in Ghana, West Africa. J Glaucoma 2002;11:416-20. [PMID: 12362081]
Medical Journal of Zambia, Vol 49, 3
The Medical Journal of Zambia, ISSN 0047-651X, is published by the Zambia Medical Association.
© This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.