Histopathological Subtypes of Primary Nephrotic Syndrome in Paediatric Patients at the University Teaching Hospital in Lusaka, Zambia
Aggrey Mweemba
School of Medicine, University of Zambia, Lusaka,Zambia
University Teaching Hospital, Children’ Hospital, Lusaka, Zambia
Department of Internal Medicine, Levy Mwanawasa University Teaching Hospital, Zambia
Paul Mashanga
School of Medicine, University of Zambia, Lusaka,Zambia
University Teaching Hospital, Children’ Hospital, Lusaka, Zambia
Department of Internal Medicine, Levy Mwanawasa University Teaching Hospital, Zambia
Justor Banda
Department of Internal Medicine, Ndola Teaching Hospital, Zambia
Department of Medical Sciences, University of Namibia
DOI: https://doi.org/10.55320/mjz.49.3.364
Keywords:Nephrotic Syndrome, Minimal Change Disease, Non-Minimal Change Disease,Focal Segmental Glomerulosclerosis
ABSTRACT
Background: Minimal change disease (MCD) has been shown to be a common histological presentation in children presenting with nephrotic syndrome (NS) in developed countries. In Africa, information on clinical and histological characteristics of paediatric NS is scarce. This study assessed the characteristics and histological subtypes of NS.
Methods: This was a prospective study that consecutively indexed children aged 2-16 years with the diagnosis of NS who were referred to the largest tertiary teaching hospital, Lusaka, Zambia. Thirteen children presenting with NS were biopsied under ultrasound guidance after ethical approval. The primary outcome of the study was to describe the histological characteristics of paediatric NS.
Results: Thirteen children of African descent with a median age of onset of NS of 9.25 years (2.0-15.0) were enrolled in the study. The histopathologic lesions were as follows; four had MCD, four had focal segmental glomerulosclerosis (FSGS), one had immune complex mediated glomerulonephritis (ICMN) and four were inconclusive. Haematuria was present in eight out of 13 patients (61.5%) and hypertension was present in five of 13 patients (38.0%). Three children did not have either haematuria or hypertension. Ten of the 13 participants had primary steroid resistance.
Conclusion: This study has demonstrated the need to perform a pre-treatment renal biopsy in paediatric patients presenting with NS in view of the atypical presentation, variable histopathologic findings, and unpredictable response to steroid therapy.
INTRODUCTION
Studies have shown that minimal change disease (MCD) is a common presentation of NS in pediatric patients.[1, 2] Information on the histological and clinical characteristics of patients with NS is still lacking in sub-Saharan Africa (SSA), Zambia included. Of note is that a few limited studies have reported non-biopsy proven NS and glomerulonephritis (GN) as the commonest glomerular diseases seen at the University Teaching Hospital (UTH), Children’s Hospital in Lusaka, Zambia.[3] NS commonly presents with oedema, hypoalbuminemia, proteinuria and hyperlipidaemia.[4, 5]
A landmark study, the international study of the primary NS (ISPNS) that indexed 521 children aged between 12 weeks and 16 years conducted in 24 clinics in North America, Europe, and Asia showed that three quarters (or 76%) of participants with NS had MCD on histology.[6] This study also assessed the response to steroids to identify children with MCD and the results revealed that, as previously viewed; children with MCD demonstrated a good response to the initial intensive course of steroids.[6] Other studies across the globe including South Africa and North Africa have demonstrated similar findings in the non-black children.[4, 7, 8, 9] Therefore, most clinicians consider children with MCD to have a very good prognosis and renal biopsy is thus only recommended in children with persistent proteinuria after eight weeks of steroids to identify those patients with different histological patterns that may have poor response to steroids.[8, 10]
However, the findings from previous studies cannot be generalized to black children from SSA as they did not include this ethnologically diverse group. Furthermore, a few African studies have shown that clinical presentation, histological patterns and steroid response may be different in African black children.[4, 7, 8, 11, 12, 13, 12, 13, 14]
In east and west Africa, secondary NS has been reported to be common complication of Plasmodium malariae infection, yet in parts of Africa where there is no malaria, MCNS is still rare with poor response to steroids.[15] Therefore, this study assessed the clinical characteristics and histological subtypes of NS in children presenting to the UTH, Children’s Hospital in Lusaka, Zambia.
METHODS
This was a prospective study in children with the diagnosis of NS aged 2-16 years who were referred to the UTHs, Children’s Hospital in Lusaka, Zambia from 2014 to 2015. The UTHs are a group of five tertiary hospitals located in the same premises and includes the Children’s hospital which was established as an independent facility in 2017. The children’s hospital is the main referral hospital for children with complex and chronic kidney disorders from the rest of the country.
Guardians/Parents of all children with the diagnosis or probable diagnosis of NS were approached to enter the study. Information about the study was given to the guardians and all children who were eligible to assent and/or whose guardians gave consent were screened for the study.
A detailed history including patient demographics , presenting complaints, past medical and drug history was taken during screening. A thorough physical examination was performed including temperature, pulse, respiratory rate, blood pressure, and urinalysis. Post examination; the would-be participants were subjected to the following investigations for safety laboratory tests to exclude secondary causes of NS: antistreptolysin O titre, hepatitis B surface antigens, hepatitis C antibody, rapid plasma reagin test for syphilis, antinuclear factor, serum complement (C3 and C4), serum albumin, serum cholesterol, serum creatinine, urea, electrolytes, liver function Tests, complete blood count (CBC), international normalized ratio (INR), bleeding time, urine and stool microscopy and HIV antibody test after pre-test counselling by qualified counsellors. Participants who met the criteria and consented for the study were recruited. A pre-biopsy abdominal ultrasound scan was performed by an experienced nephrologist on patients that met the criteria for renal biopsy.
The renal biopsies were ready by an experienced pathologist using light microscopy with several stains. Analysis for immunofluorescence or electron microscopy was not feasible due to budget limitations.
NS was defined as the presence of oedema, massive proteinuria (>40 mg/m2/hr or a urine protein/creatinine ratio >2.0 mg/mg), and hypoalbuminemia (<2.5 g/dL).[4, 16, 17] In this study, a dipstick proteinuria of 3+ or more was considered significant.
- Remission was defined by a marked reduction in proteinuria (urine albumin dipstickof 0 to trace for 3 consecutive days) in association with resolution of oedema and normalization of serum albumin to at least 3.5 g/dL.[17]
- Steroid-sensitive nephrotic syndrome (SSNS): Children whose proteinuria resolved for 3 consecutive days after 6-8 weeks of daily steroid therapy.[1]
- Steroid-Resistant Nephrotic Syndrome (SRNS): Patients who fail to enter remission after 8 weeks of corticosteroid treatment.[17]
- Hypertension was defined as elevated systolic and/or diastolic blood pressure above the 95 th percentile for age, gender, and height.[13]
Statistical Analysis:
Data analysis was performed using Stata version [13] . Descriptive statistics comprising means, standard deviation (SD), median, percentages, and proportions depending on data skewedness were utilized.
RESULTS
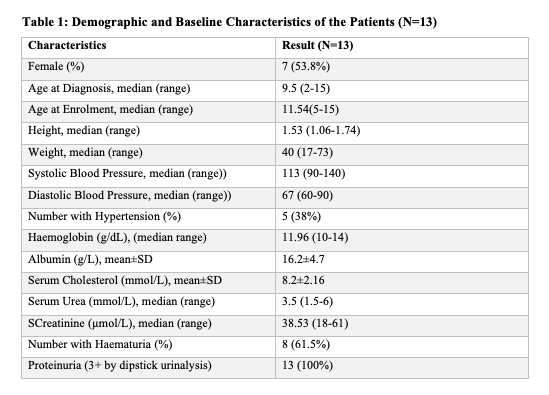
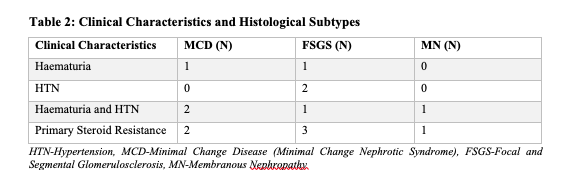
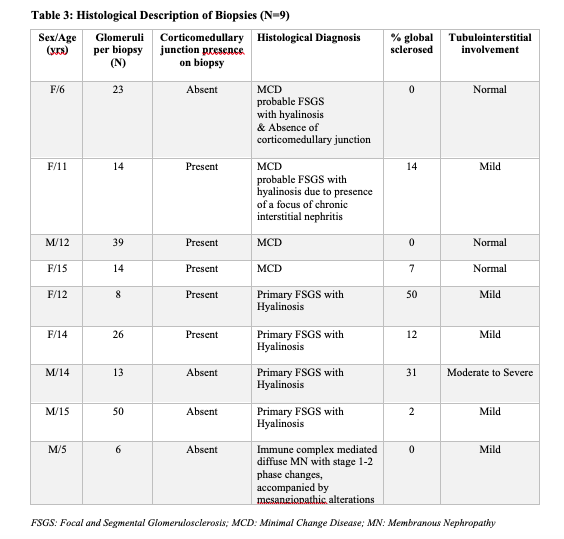
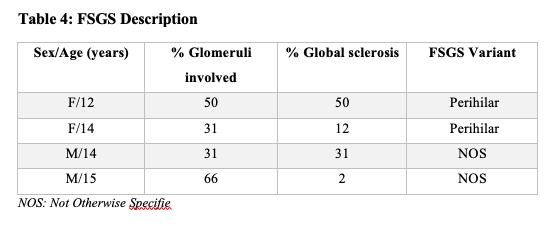
A total of thirteen children of African descent were enrolled in this study with a median age of onset of NS of 9.25 years (IQR 2.0-15.0) and seven out of 13 were females (Table 1). The histopathologic lesions were as follows (Table 3); four had Minimal Change disease (MCD), four had Focal Segmental Glomerulosclerosis (FSGS) and one had immune complex mediated Membranous Nephropathy (ICMN). Histology reports for the other four participants were inconclusive. Two patients with FSGS had the perihilar variant while the other two had the not otherwise specified (NOS) variant (Table 4).
Of the four participants with FSGS, one had moderate to severe tubulointerstitial involvement, and the rest had mild tubulointerstitial involvement. Two patients with FSGS had more than 50.0% glomeruli affected. Global sclerosis ranged between 2.0% to 50.0% among participants with FSGS.
Ten out of the thirteen patients had atypical presentation at diagnosis (Table 1). Haematuria was present in eight out of 13 patients (61.5%) and hypertension was present in five of 13 patients (38.0%). Three and all the four patients with MCD and FSGS respectively had either haematuria or hypertension or both (Table 2). Only three out of 13 participants did not have either haematuria or hypertension.
The one FSGS patient who went into remission had perihilar variant with without tubulo-interstitial involvement nor globally sclerosed glomeruli.
Seventy seven percent of the participants had primary steroid resistance; three among the MCD participants, three among the FSGS participants, one participant with ICMN and three among those with inconclusive histological diagnosis.
DISCUSSION
Our results show a very different histological pattern from the ISPNS, which showed that 77.4% of children had MCD, 7.5% with Membranoproliferative (MPGN) and only 6.9% had FSGS.[13] These results are consistent with other findings in SSA which show diverse histological patterns. In Durban, South Africa, Bhimma et al. reports low prevalence of MCD at 13.5% (with only 12 with SS), FSGS at 28.4% and high prevalence of MN at 40.0% among 236 South African black children.[18] In Zimbabwe, the prevalence of MN associated with Hep B infection was reported to be the commonest while in Ghana, Adu et al. reports a 56.0% (14/25)of MCD and that FSGS was also a common histological pattern among children with NS.[11, 12]
Children with NS in our study presented with atypical clinical features that included haematuria (61.5%), hypertension (38.0%) and a median age of onset of 9.25 years. All the nine children with a histological diagnosis were aged ≥6 years except for one child who was 2 years old. This contrasts with the ISPNS, in which; 80.0% of the 398 children witha histological diagnosis of MCD, 50.0% of the 36 childrenwith FSGS and 2.6% of the 39 children with MPGN were below 6 years of age.[2, 18] In the South African study by Bhimma et al., the peak age of onset among the black children with NS (MCD, MPGN, FSGS, and membranous) was 5-8 years and 2-4 among the Indian children.[18] The high frequency of atypical presentation in our study (i.e. age at presentation, haematuria and hypertension) may reflect non-MCD predominance.
The proportion of children with hypertension was also different in our study from the ISPNS. In the ISPNS, systolic BP above the 98th percentile was noted in only 21% among those with MCD, but 48.5% and 51.4% among those with FSGS and MPGN respectively.[2] However, in our study the number of children with hypertension was high; 50.0% (2/4) among MCD and 75.0% (3/4) among FSGS. Similar to our study, Bakhie et al. from South Africa recorded 47.0% hypertension among children with NS except this finding was attributed to the higher rate of FSGS (43.2%).[12] Whereas we report a high proportion of haematuria among MCD (75.0%) and FSGS (50.0%), haematuria in the ISPNS was only 23.0% (80/356) among those with MCD, and higher among those with FSGS (48.4%), and MPGN (59.0%).[2]
Five out of the nine participants who had a definitive histopathologic diagnosis in our study had non-MCD. Additionally, two participants with MCD had features of FSGS (hyalinosis with a focus of chronic interstitial nephritis) and FSGS could not be completely ruled out. However, the fact that all the four children with MCD (including two suspected FSGS) were aged 6 years, 50.0% (2/4) had hypertension and 75.0% (3/4) had hematuria at initial presentation, may suggest that MCD has an atypical presentation in this population. Therefore, it makes it very difficult to distinguish MCD from non-MCD in these children based on clinical features.
Only three of the 13 participants had remission during the study period and primary steroid resistance was very high in both MCD and FSGS participants. This may reflect misdiagnosis of FSGS among the four participants classified as MCD. Notable is that two of the four participants with MCD had some features suggestive of FSGS.
Primary steroid resistance may also indicate inherent resistance to steroids among the black children with NS as previously shown elsewhere.[5, 18, 19] In a South African study, only 14.0% of the black and 52.0% of the Indian children with NS responded to steroids.8 In some studies, atypical presentation like hematuria, hypertension, ethnicity, and older age were used to predict outcomes and distinguish between histology patterns.[2, 20, [21] However, some limited studies have shown that atypical presentation may not be a determinant of poor prognosis.[8, 22]
The predominance of non-MCNS and the atypical presentation of MCD among children of African descent makes pre-treatment kidney biopsy important. This recommendation is supported by authors of the Nigerian study who also recommended routine pre-treatment renal biopsy.[13] Pre-treatment kidney biopsy would help tailored treatment for different histopathologic patterns.
The small sample size limits generalisability of the findings to the rest of the country or the region. Light microscopy alone was not adequate to give more detailed histological analysis of the two children who were classified as MCD. Immunofluorescence and electron microscopy studies would have given more detail, however, due to financial constraints, this was not possible.
In conclusion, this study has demonstrated the need to perform a pre-treatment renal biopsy in paediatric patients presenting with NS in view of the atypical presentation, variable histopathologic findings, and unpredictable response to steroid therapy. We recommend a larger study be done, which should include immunofluorescence and electron microscopy, to describe the histological subtypes better.
DISCLOSURE
Financial Disclosure:
This study had no source of funding other than the principle author and therefore there were no conflicts of interest, nor influence of outcomes associated with any financial sources.
Competing interests:
The listed authors have no competing interests
Authors’ contributions
All the listed authors AM, PM and JB contributed to the journal in line with International Committee of Medical Journal Editors (ICMJE) guidelines.
Acknowledgements
The authors acknowledge Professor Mary Shilalukey Ngoma and Dr Veronica Mulenga for the contribution.
Ethics approval
Permission to perform the study was granted by the ERES CONVERGE IRB (ERES ref# 043) and the local hospital leadership
REFERENCES
- Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal Change Disease. Clinical Journal of the American Society of Nephrology : CJASN, 2017. 12(2): p. 332-345.
- Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int, 1978. 13(2): p. 159-65.
- Shilalukey-Ngoma M, Shakankale GM, Mutiti A, Mutale W, Sinyinza F. The challenge of providing tertiary care for renal disease in children admitted to the University Teaching Hospital, Lusaka Zambia. Medical Journal of Zambia, 2007. 34(1): p. 36-41.
- Abumregha O, Naicker E, Connolly C, Bhimma R. Primary nephrotic syndrome in the new millennium in KwaZulu-Natal, South Africa. South African Medical Journal; Vol 110, No 7 (2020), 2020.
- Gulati S, Sengupta D, Sharma RK, Sharma A, Gupta RK, Singh U et al., Steroid resistant nephrotic syndrome: role of histopathology. Indian Pediatr, 2006. 43(1): p. 55-60.
- Pokrajac, D., A.H. Kamber, and Z. Karasalihovic, Children with Steroid-Resistant Nephrotic Syndrome: A Single -Center Experience. Materia socio-medica, 2018. 30(2): p. 84-88.
- Bakhiet YM, Mudi A, Khumalo T, Moonsamy G, Levy C. Idiopathic nephrotic syndrome in South African children. Afr Health Sci, 2017. 17(4): p. 1130-1136.
- Elzouki AY, Amin F, Jaiswal OP, Primary nephrotic syndrome in Arab children. Arch Dis Child, 1984. 59(3): p. 253-5.
- Mortazavi F, Khiavi YS. Steroid response pattern and outcome of pediatric idiopathic nephrotic syndrome: a single-center experience in northwest Iran. Ther Clin Risk Manag, 2011. 7: p. 167-71.
- Gulati S, Sharma AP, Sharma RK, Gupta A, Gupta RK. Do current recommendations for kidney biopsy in nephrotic syndrome need modifications? Pediatr Nephrol, 2002. 17(6): p. 404-8.
- Adhikari M, Coovadia HM, Chrystal V. Extramembranous nephropathy in black South African children. Ann Trop Paediatr, 1983. 3(1): p. 17-24.
- Adu D, Anim-Addo Y, Foli AK, Blankson JM, Annobil SH, Reindorf CA, et al. The nephrotic syndrome in Ghana: clinical and pathological aspects. Q J Med, 1981. 50(199): p. 297-306.
- Seggie J, Davies PG, Ninin D, Henry J. Patterns of glomerulonephritis in Zimbabwe: survey of disease characterised by nephrotic proteinuria. Q J Med, 1984. 53(209): p. 109-18.
- Sorof JM, Hawkins EP, Brewer ED, Boydstun, II, Kale AS, Powell DR. Age and ethnicity affect the risk and outcome of focal segmental glomerulosclerosis. Pediatr Nephrol, 1998. 12(9): p. 764-8.
- Hendrickse, R., Epidemiology and prevention of kidney disease in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene, 1980. 74(1): p. 8-16.
- El Bakkali L, Rodrigues Pereira R, Kuik DJ, Ket JC, van Wijk JA. Nephrotic syndrome in The Netherlands: a population-based cohort study and a review of the literature. Pediatr Nephrol, 2011. 26(8): p. 1241-6.
- Obiagwu P, Aliyu A, Atanda A. Nephrotic syndrome among children in Kano: A clinicopathological study. Nigerian Journal of Clinical Practice, 2014. 17(3): p. 370-374.
- Bhimma R., Coovadia HM, Adhikari M. Nephrotic syndrome in South African children: changing perspectives over 20 years. Pediatr Nephrol, 1997. 11(4): p. 429-34.
- Olowu W, Adelusola K Adefehinti O. Reversed clinical and morphologic characteristics of idiopathic childhood nephrotic syndrome. the journal of nephro-urology monthly, 2(1), 200-211, 2010. J Nephro-Urol Monthly, 2010. 2(1), 200-11.
- Imbusi EA, Ekanem PE, Gebrearegay H, Ambaye M, Tesfahunegn A, Nyaga K et al., Steroid response pattern among children with nephrotic syndrome in Northern Ethiopia. Nephro-Urology: 2020, Vol.12, issue 4; e106995
- Begum A, Rahman H, Hossain MM, Sultana A, Jahan S, Muinuddin G. Histological variant of nephrotic syndrome with atypical presentation in children. Mymensingh Med J. 2009 Jan;18(1):42-6.
- Gooden M , Miller M, Shah D, Soyibo AK, Williams J, Barton EN. Clinicopathological features of atypical nephrotic syndrome in Jamaican children. West Indian Med J. 2010 Jun; 59(3):319-24.
Medical Journal of Zambia, Vol 49, 3
The Medical Journal of Zambia, ISSN 0047-651X, is published by the Zambia Medical Association.
© This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.