In vivo Anti-anaemic Effect of an Aqueous Root Extract of Phyllanthus muellerianus (Kuntze) Exell in Model Rats.
Authors
James Nyirenda
University of Zambia, School of Natural Sciences, Department of Chemistry, P.O. Box 32379 Lusaka, Zambia.
Gershom B. Lwanga
University of Zambia, School of Medicine, Department of Physiological Sciences, P.O. Box 32379 Lusaka, Zambia
Kaampwe M. Muzandu
University of Zambia, School of Veterinary Medicine, Department of Biomedical Sciences, P.O. Box 32379, Lusaka, Zambia.
David K. Chuba
University of Zambia, School of Natural Sciences, Department of Biological Sciences, P.O. Box 32379 Lusaka, Zambia.
Gibson M. Sijumbila
5School of Medicine and Health Sciences, Mulungushi University, P.O. Box 6009, Livingstone, Zambia
DOI: https://doi.org/10.55320/mjz.49.3.357
Keywords:Haematological indices, Phyllanthus muellerianus, Phytochemicals,Indigenous Traditional Knowledge Systems
ABSTRACT
Background: Anaemia is a very serious condition in Zambia. One of the plants that has been used traditionally is Phyllanthus muellerianus where different parts of shrub are used to treat a number of diseases in Zambian folklore medicine. Earlier studies have investigated medicinal properties of its aqueous root extracts. This study evaluated the effect of P. muellerianus roots on the haematological indices of albino rats and determined its phytochemical profile. In order to prove this, we carried out phytochemical screening of the root extract and assessed the anti-anaemic effect of the aqueous extract on laboratory rats with tail-bled induced anaemia.
Materials and Methods: Thirty-six male albino rats placed in six groups were used for the study. The groups comprised the 100, 200, and 400 mg/kg plant extract, Ranferon (200 mg/kg positive control), anaemic (non-treated control) and a normal (non-anaemic control) groups. Anaemia, induced through bleeding of the rats, was defined as haemoglobin (Hb) levels less than 12 g/dL. The anti-anaemic potential of the plant was determined by comparing its effect on the haematological parameters of rats on treatment to that of the control group.
Results: Phytochemical screening revealed positive results for alkaloids, flavonoids, saponins, glycosides, steroids, triterpenoids and tannins with varying amounts. After treatment, rats on the 400 mg/kg plant extract dose showed the greatest increase in the mean values for Hb, Packed cell volume (PCV) and RBC count were 43.3±1.2%, 15.4±0.3 g/dL and 6.3±0.3 x106 /L respectively, when compared to the negative control group (P < 0.05).
Conclusions: The aqueous root extract of P. muellerianus was efficacious against anaemia in a dose-dependent manner. The phytochemical compositions seem to be responsible for its haematopoietic properties. Thus, the root decoction of P. muellerianus is useful in alleviating anaemia and the results lend credence to its use in traditional medicine in the management of anaemia.
List of abbreviations
- AAS - Atomic Absorption Spectrophotometer/Spectrophotometry
- ANOVA - Analysis of Variance
- Hb - Haemoglobin
- MCH - Mean Corpuscular Haemoglobin
- MCHC - Mean Corpuscular Haemoglobin Concentration
- MCV - Mean Corpuscular Volume
- PCV - Packed Cell Volume
- RBC - Red Blood Cell
- SD - Standard Deviation
- UNZABREC - University of Zambia Biomedical Research Ethics Committee
- UZL - University of Zambia Herbarium
INTRODUCTION
Pyllanthus muellerianus is one of the plants used to treat anaemia by local people of the Northern part of Zambia. However, its efficacy has not been scientifically established. Local names for P. muellerianus in Zambia include: Chewa-Mkuzandola, Tumbuka-Kapikanduzi,[1] Icibemba-Umupetwalupe, Kaonde-Mulembalemba, Mambwe-Mupetwandupe. It belongs to the family Phyllanthaceae consisting of approximately 1000 species which are widely distributed in tropical and subtropical areas of Africa, Asia, America and Australia. It is an evergreen scandent shrub with numerous stems from the base or a small tree up to 12 metres tall. The branches are arched and pendulous almost to the ground. It naturally occurs in riverine forest and wooded grasslands on deep and well-drained soils. It is widely distributed and easy to access in Zambia. Additionally P. muellerianus grows easily from seed and hence has potential to contribute sustainably towards medical solutions coming from local flora.[2] P. muellerianus has many medicinal uses such as the treatment of wounds, menstrual disorders, fevers, inflammation, intestinal problems, kidney and urinary bladder problems, diabetes and hepatitis B, body pain and as an antiseptic.[3] Agyare et al[4] studied P. muellerianus leaf extracts for the stimulatory effect of ellagitannins on cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Earlier studies on the leaf extract by Boakye et al [5] have shown that P. muellerianus has anti-inflammatory activity. The review by Calixto and friends[6] showed that the phyllanthus species have a number of metabolites with pharmacological potential isolated and characterised from all the parts of the plant, leaves, roots, stem and bark. Other studies investigated the antimicrobial properties of the stem and bark parts.[7, 8]
Anaemia is a condition in which the number of red blood cells (and consequently their oxygen-carrying capacity) is insufficient to meet the body’s physiologic needs. [9-11] The cut off points for diagnosis of anaemia have remained largely unchanged since 1968 with the exception that the original age group of children 5-14 years of age was split, and a cut-off of 0.5 g/dL lower was applied to children 5-11 years of age to reflect findings among non-iron deficient children in the USA.[12] The cut offs range from children 6-59 months (10-10.9 g/dL), children 5-11 years (11-11.4 g/dL), children 12-14 years (11-11.9 g/dL), non-pregnant women 15 years and above (11-11.9 g/dL), pregnant women (10-10.9 g/dL) and men 15 years and above (11-12.9 g/dL).[9, 13] It is a common blood disorder that affects people of all ethnicity and ages; although the elderly, young women of child bearing age and infants at greater risk [14] . The causes of anaemia are patho-physiologically diverse and multifactorial. Thus, there are more than 400 types, some are mild while others are severe or even life threatening if not treated [15] . In rodents, symptoms include; rapid or laboured respiration, anorexia, immobility, abnormal appearance or posture periocular and nasal porphyrin discharge.[16] Treatment varies depending on the type of anaemia. Anaemia associated with a serious disease is treated by treating the underlying disorder. Additional medications that boost red blood cells (RBC) may be prescribed when symptoms persist or worsen. These include; iron tablets, supplements, fortifications, erythropoietin injections, blood transfusions, removal of the spleen, plant products such as groundnuts, tomatoes and spinach, animal products such as liver and red meat[13, 17, 18] etc. Interventions to prevent or treat anaemia are insufficient in Zambia because of inadequate qualified human resource, high disease burden, inadequate emergency facilities, the diet of majority Zambians is mainly composed of cereals (maize) and starchy roots with little micronutrient-dense foods such as animal products and fruits [19, 20] . Further, the prevalence of anaemia is 46 % which is a severe public health problem based on the World Health Organization (WHO) standards. This implies that there is a great loss of man hours of healthy adults who find themselves off work to nurse anaemic patients. It is also among the top 10 causes of morbidity and mortality.[12, 21-24] In Africa and most Asian countries, anaemia is treated using herbs such as Khaya senegalensis, Justicia secunda, and Amaranthus spinosus.[25] The study investigated the effect of Phyllanthus muellerianus (Kuntze) Excell aqueous root extract on haematological indices of albino rats.
MATERIALS AND METHODS
Study design and sample size
This study was experimental development, drawing on from indigenous knowledge and practical experience. Specifically, the crude extract of P. muellerianus was evaluated. and sample size was calculated by executing the function in G*Power version 3.1.9.4 [26, 27] . Briefly, under test family, “F test” was selected, and the statistical test selected was “ANOVA: Fixed effects, omnibus, one-way”. Sample size was then computed as a function of power level (1 - β), a pre-specified significance level (α) and the population effect size to be detected with probability. The power level (1 - β) was set at 0.8 default, significance level α at 0.05 default and pre-detected effect size at 0.7, and 6 groups. Calculation using G*Power gave a sample size of 36. So, a total of 36 male albino rats (Rattus norvegicus) weighing between 150- and 180-grams body weight with no previous drug treatment, were acquired from the Animal House, Department of Biological Sciences, School of Natural Sciences, University of Zambia. Anaemia was induced by bleeding the rats under light anaesthesia using halothane as an anaesthetic.[28] The formula described by Lee and Blaufox[29] was used to determine the quantity of blood removed through bleeding. At the end of the study, rats were euthanized by use of sodium pentobarbital.[28]
Plant Collection and Identification
Ethnobotanical authentication and annotation were done at the University of Zambia Herbarium (UZL). The specimen was identified as Phyllanthus muellerianus (Kuntze) Exell belonging to the family Phyllanthaceae and voucher specimen/accession number 22287. It has also been verified on the http://www.theplantlist.org website.
Preparation of the aqueous root extract of the plant
The roots of the plant were harvested in October 2018 from Kaunda Square area of Lusaka Province (Latitude 15°21'33.1"S and Longitude 28°21'57.9"E), Zambia. They were thoroughly washed to remove debris and all soil material. After size reduction, a 1 kg root sample was boiled in 1.5 litres of distilled water and the resulting solution was allowed to cool then sieved to remove non-soluble plant matter and finally filtered through Whatman filter paper number 4. The mixture was boiled to dryness on a heating mantle, the resulting brittle powder was weighed and kept at 4 oC until further use. Various concentrations (100, 200 and 400 mg/kg) of the extract per 1 mL solution were made by dissolving the appropriate quantity of the solid extract in distilled water to make a solution. The percent yield was calculated by dividing the starting material by the amount of extract. The plots were generated using GraphPad Prism software version 6.01.
Experimental animals and inclusion criteria
The rats were taken to the Animal House at the School of Veterinary Medicine, University of Zambia and allowed to acclimatize for a period of 7 days before treatment. They were maintained under standard animal house conditions (temperature: 26–30 °C, photoperiod: approximately 12 h natural light per day and relative humidity of 55–60 %) with continuous access to pelleted food and water. The rats were put into 6 treatment groups with 6 animals each using similar studies.[30] All laboratory work was done according to the Guidelines for the Care and Use of Laboratory Animals.[16] Thirty six animals (n=36) were used in this study. The adult rats were of the same age and same genetic make-up (genetic type not supplied but backcrossing was confirmed for more than 15 generations). The weight range selected was 150-180 g and all rats were tested for absence of anaemia before start of treatments. Only male rats weighing between 150-180 g were used in this study. Rats with an initial haemoglobin level of more than 12 g/dL were selected for the study.
Randomization and Dosing
Rats were randomly assigned to the six groups namely: negative control, normal control, Ranferon, 100 mg/kg, 200 mg/kg and 400 mg/kg respectively. This was done by utilizing the Research Randomizer version 4.0 online software.[31] Blinding was done at allocation and during treatment only. Only one researcher had access to treatment groups. Treatment started 24 hours after inducing anaemia. Briefly 1.0 mL each of Ranferon, the extract and distilled water were administered by oral intubation for 10 days[32, 33] to each animal bearing in mind the mean weight per group which was used to normalize the concentration for each treatment. Rats were anaesthetised in diethyl ether, when they became unconscious, blood was collected from their retro-orbital plexus for haematological studies [34] at the baseline of the study, after inducing anaemia and after treatment [35, 36] .
Phytochemical Screening
Qualitative phytochemical screening of the aqueous plant extract was carried out at the University of Zambia, Department of Chemistry using standard procedures.[37, 38]
Outcome measures
The following parameters were assessed: PCV, Hb amount, HCB, MCHC and RBC count.
Statistical methods
One-way analysis of variance (ANOVA) followed by Bonferroni post hoc test to determine pairwise comparisons was executed in STATA software version 13.0. In each case, the negative control was compared with other categories. All results were expressed as percent means at 95% confidence level with respective standard deviations.
Mineral quantification and Quality control analysis
The mineral content was determined by AAS using Perkin-Elmer atomic analyst 400.To ensure reliability of results, quality control was performed on the AAS and haematocrit before each test was done according to the respective manufactures manual.
RESULTS
The extractive weight of the plant extract was 16 g with a mineral composition of 230.5 mg Fe, 273.5 mg Mn, and 138 mg Zn. The colour of solid extract was dark brown and the percentage yield was 1.6 % calculated from the initial amount of 1 kg sample.
Phytochemical Screening
The results of phytochemical screening of the extract are shown in Table 1.
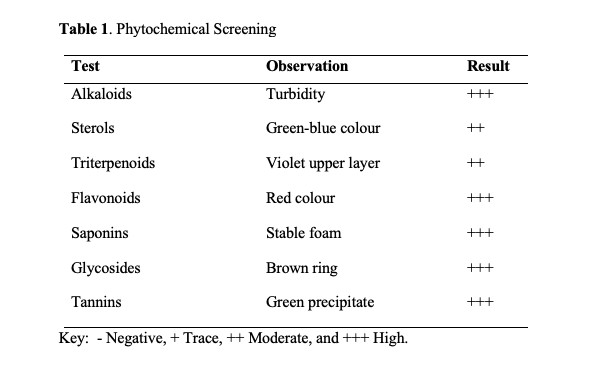
Effects of the plant extract on the Packed Cell Volume and Haemoglobin
The study defined anaemia as Hb < 12 g/dL. At the baseline of the study, the mean values for PCV and Hb in the 5 comparison groups were not different (p > 0.05) from the control group. However, repeated bleeding decreased PCV and Hb to values less than 32.5 % and 11.5 g/dL respectively. Treatment with P. muellerianus reversed anaemia in a dose-dependent manner (Figure 1). The mean PCV for the negative control was 35.5±0.84 % significantly lower than the means of normal control 42±1.7%, Ranferon 42±0.0%, 100 mg/kg (40.8±0.8%), 200 mg/kg (43.3±1.2%) and 400 mg/kg (46.2±1%) all at p-value <0.05 as shown in Figure 1. The normal control was significantly lower than the 400 mg/kg, Ranferon was lower than 400 mg/kg, 100 mg/kg to 200 mg/kg, 100 mg/kg to 400 mg/kg and 200 mg/kg to 400 mg/kg.
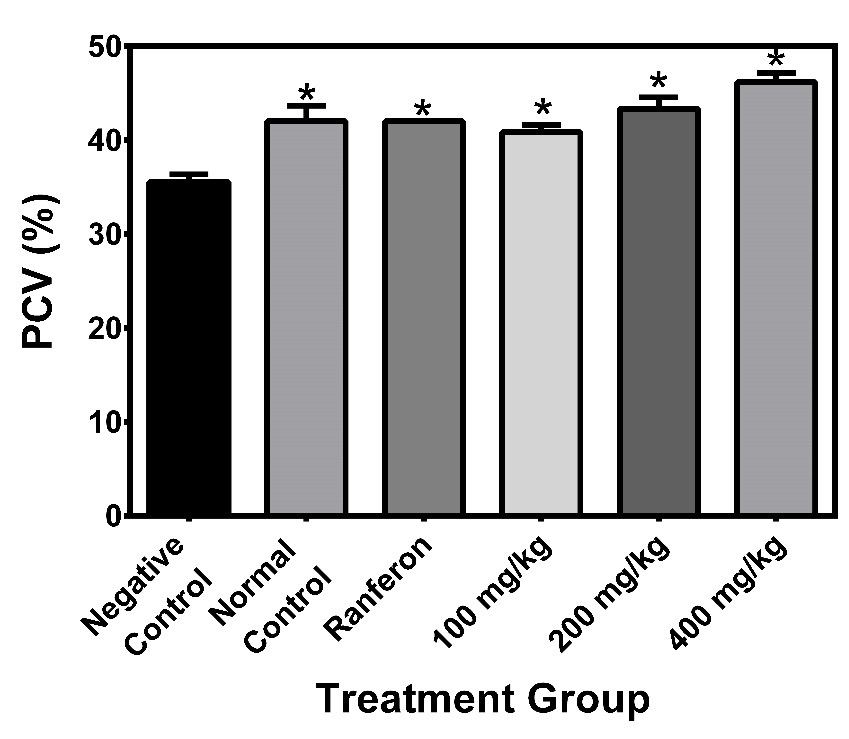
Effects of the extract on Hb levels
The mean haemoglobin levels for the negative control, normal control, Ranferon, 100 mg/kg, 200 mg/kg and 400 mg/kg were 11.8±0.3, 13.9±0.6, 14.0±0.0, 13.6±0.3, 14.5±0.4 and 15.4±0.3 respectively. Analysis showed statistical differences across the groups (p=0.03). The negative control was significantly lower compared to all other groups. There were also differences when the normal control was compared to 400 mg/kg. Ranferon was lower compared to 400mg/kg, 100mg/kg was lower compared to 200 mg/kg and 400 mg/kg. Also, the 200mg/kg was lower when compared to the 400 mg/kg extract (Figure 2).
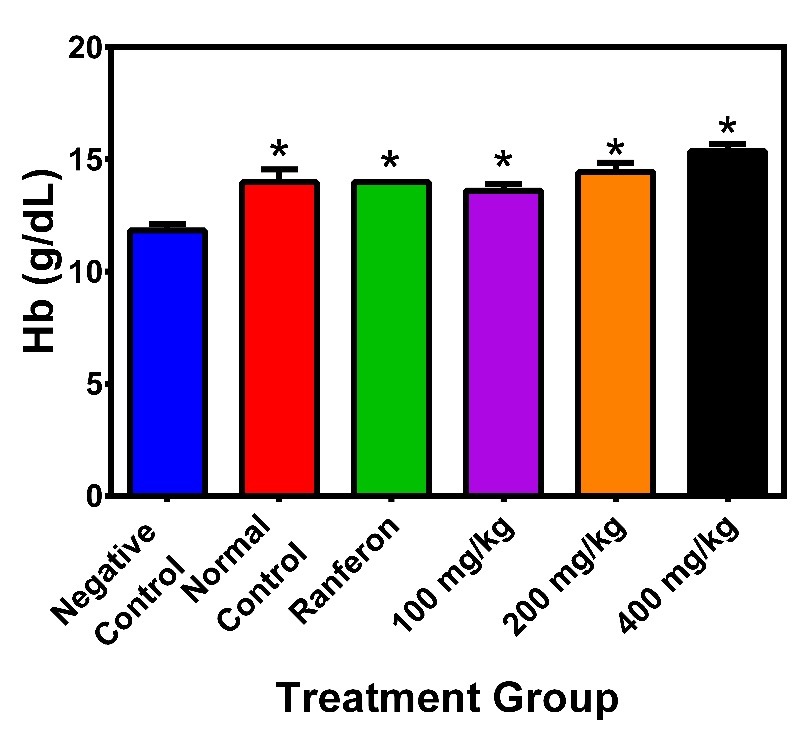
Effects of the plant extract on RBC Count
The mean values of RBC count ranged from, 5.3 to 5.9 x 106 /µl (p > 0.05) at the baseline of the study, 3.9 to 5.7 x 106 /µL after inducing anaemia, and 4.9 to 6.3 x 106 /µL after treatment, p < 0.05. RBC mean values for the negative control, normal control, Ranferon, 100 mg/kg, 200 mg/kg and 400 mg/kg were 5.1±1.4, 5.1±0.2, 5.1±0.1, 4.9±0.6, 5.9±0.9 and 6.3±0.3 respectively. One-way ANOVA showed statistical differences across the groups (Figure 3).
Effects of the plant extract on MCV, MCH and MCHC
The mean corpuscular volume (MCV; p=0.34), mean corpuscular haemoglobin (MCH;p=0.31) and the mean corpuscular haemoglobin concentration (MCHC;p=0.25) all showed no statistical differences across the groups at 95 % confidence level.
MCV and MCH
Means of the Negative control (74.7±20.2), Normal control (81.8±0.9),Ranferon (82.4±1.0),100 mg/kg (83.7±9.2), 200 mg/kg (75.2±9.8) and 400 mg/kg (73.8±3.4) for the corpuscular volume showed no statistical significance between and across the treatment groups (Figure 4 a). Means of the Negative control (24.9 ± 6.7), Normal control (27.2 ± 0.3), Ranferon (27.4±0.3), 100 mg/kg (27.8 ±3.1), 200 mg/kg (24.9 ±3.3) and the 400 mg/kg (24.6±1.2) treatment groups showed no statistical significance across the treatment groups (Figure 4 b).
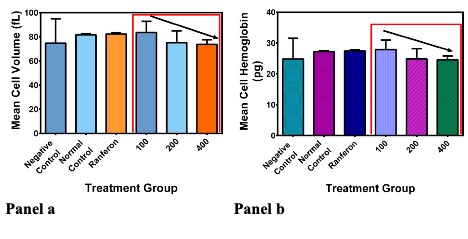
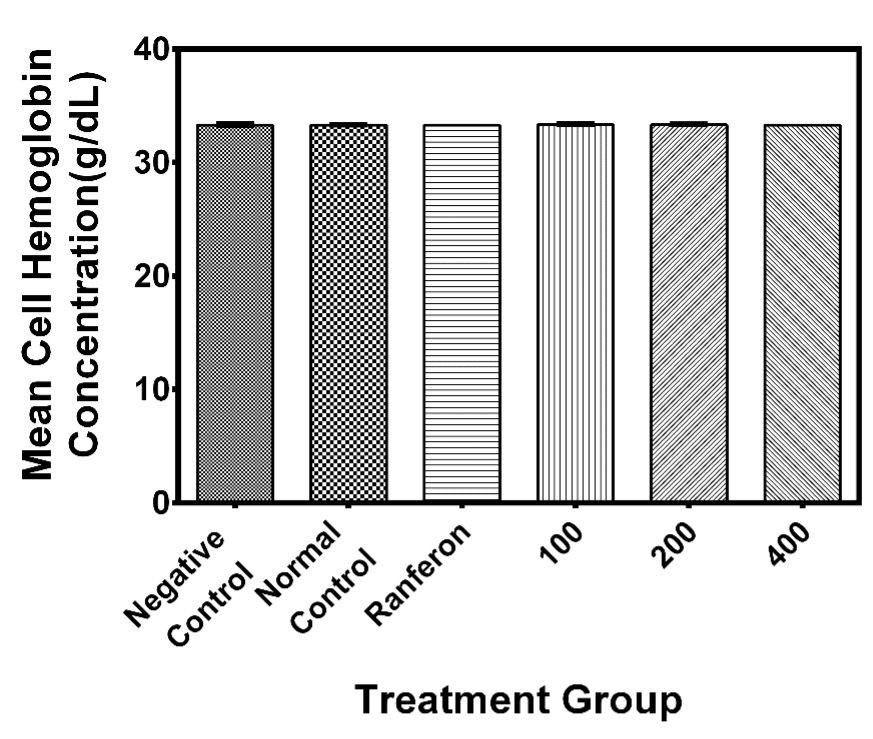
MCHC
The mean cell haemoglobin concentration was Negative control (33.3±0.06), Normal control (33.3±0.04), Ranferon (33.3±0), 100 mg/kg (33.4±0.05), 200 mg/kg (33.3±0.05) and the 400 mg/kg (33.3±0) in g/dL units respectively. Figure 5 shows the MCHC plots verses treatment groups. There was no statistical difference (p=0.25) for all the treatment groups.
DISCUSSION
Effect of P. muellerianus on the haematological parameters
Dosages of 100, 200, and 400 mg/kg of the root extract and ranferon were administered orally to anaemic albino rats to monitor their effect compared to that of the control group that did not receive any drug but distilled water. The results revealed that the root extract and Ranferon were able to restore the haematological indices of experimental animals to normal levels and the significant (p < 0.05) effect was found to be dose-dependent. In addition, the eta squared values for PCV and Hb of 0.66 suggested that the aqueous root extract of the plant was both statistically significant and efficacious against anaemia. The Phytochemical and mineral compositions of the root extract seem likely to be responsible for the haematinic effect of P. muellerianus and their presence in the plant extract agrees with previous studies.[39, 40] The blood parameters, Hb, PCV, and RBCs together with the level of iron are indices of anaemia that could be used to indicate nutritional values of ingested diets as well. MCV, MCH and MCHC are constants for typing anaemia hence they were not statistically different (p > 0.05) when experimental groups were compared to the control group after treatment. They, however, decreased after inducing anaemia, indicating microcytosis as their decrease reflects a release of RBCs, which are less saturated in Hb (hypochromia). Therefore, occurrence of anaemia observed in this study was attributable to lowered values of these indices and related to the report by Osman et al.[41] Moreover, the increase in these haematological parameters was dose-dependent and could be due to high nutritional values of P. muellerianus particularly in minerals such as Fe (230.5 mg). Fe plays a significant role in erythropoiesis. It is required for the synthesis of Hb and myoglobin while its deficiency causes anaemia. However, the therapeutic potential of P. muellerianus could not be established based on available Fe content alone as other factors play a role in its absorption in the body. In this context, Fe is a necessity for the formation of the heme part of Hb as reported by others.[15] The high Fe content of the plant under investigation justifies and partly supports the traditional use of its roots in treating anaemia. The results also place it under a group of plants with anti-anaemic potential, which are richest in Fe content according to the related findings of Koné et al.[25] However, Schmelzer et al.,[3] reported an insignificant Fe content of 15 mg per gram of dry fruits of P. muellerianus. The plant extract also had high content of Zinc (138 mg) and Manganese (273.5 mg). Zinc is required for the function of over 200 enzymes and is important in growth and sexual development in man. Mn is both nutritionally essential and potentially toxic. It is important for brain and nerve function (can bind with neurotransmitters and stimulate faster or more efficient transmission of electrical impulses throughout the body, in effect, speeding up cognitive function.15 The methanolic and ethyl acetate leaf extracts of P. muellerianus were shown by Assob et al.,42 to have a lethal dose (LD 50) of more than 4 g/kg body weight of male and female rats. Based on their study, they concluded that P. muellerianus is not toxic, taking into consideration the 5 g/kg threshold of toxic substances. On the other hand, Adedapo et al.,[43] showed that the leaves of P. muellerianus significantly (p < 0.05) reduced the haematological parameters, had toxic potential and were therefore, poisonous to the animals.
Observed Phytochemicals and their action
Plants used in the treatment of disease contain a wide range of active principals with biological activity, some of which are responsible for the characteristic odours, pungencies and colours of plants while others give a particular plant its culinary, medicinal or poisonous virtues, which could be used as the base for discovering modern drugs for curing various diseases.[39] The phytochemical tests performed on the root extract of P. muellerianus showed presence of steroids, triterpenoids, alkaloids, flavonoids, saponins, cardiac glycosides and tannins as reported in previous similar studies.[3, 39, 44]
Sterols
Phytosterols (PS) such as β-sitosterol, campesterol and stigmasterol[45] have been shown to have anti-eryptotic effects on cells. Presence of a considerable number of sterols may have had the anti-haemolytic property hence improving availability of intact cells in blood, ultimately alleviating anaemia.
Alkaloids
As inferred from other reports, alkaloids, the most revered of all phytochemicals, are said to be pharmacologically active and their action is felt in blood vessels. They inhibit cyclic adenosine monophosphate (cAMP) phosphodiesterase leading to accumulation of cAMP. This effect stimulates phosphorylation of proteins and synthesis of proteins, which improves erythropoiesis.[39, 40]
Saponins
Saponins are known to vitalize blood circulation and promote haemolytic activities. Since saponins are active agents that lyse the membrane of RBCs, it is likely that the plant extract used in this study first lysed RBCs. Then the test animals overcame this inhibition by producing a glycosidic enzyme that cleaves some of the terminal sugars from the saponins, which detoxified it.[39] This detoxification of saponins reinforced the proper use of iron contained in the plant extract allowing it to synthesize haemoglobin for new RBCs, thus leading to an observed improvement of Hb, RBCs and PCV in the plant extract treated groups. Glycosides enhance the natural resistance and have the recovery powers for the body.[39]
Flavonoids
Flavonoids are known to possess a well-established protective effect against membrane lipoperoxidative damages. The antioxidant activity of phenols and flavonoids is mainly attributed to their redox properties because of which they act as reducing agents, electron/ hydrogen donators, and singlet oxygen quenchers. It has been demonstrated that the antioxidants such as flavonoids can act: either by neutralizing reactive oxygen species (ROS) by directly reacting with superoxide anion, nitric oxide and peroxynitrite thereby preserving vascular function and protecting vascular injuries from ROS and perhaps from other oxidant species, or they could stimulate erythropoiesis.[46]
Tannins
Tannins are well known for their anti-oxidant and skin regeneration. Tannins from many plants especially Euphorbiaceae are used to stop bleeding during circumcision. Steroids regulate carbohydrate and protein metabolism and possesses anti-inflammatory properties. This might correspond to their ethnomedicinal significance.[39]
P. muellerianus was efficacious against anaemia in a dose dependent manner, p < 0.05. The rich array of phytochemicals (especially alkaloids, saponins, and flavonoids) and high iron composition seem likely to be responsible for its haematinic effect. Further studies are needed with this plant to evaluate the anti-anaemic active components and to elucidate their mode of action.
CONCLUSION
Our study revealed that P. muellerianus was efficacious against anaemia in a dose dependent manner, p < 0.05. This information further enhanced the ethnobotanical and ethnomedicine information already possessed as indigenous knowledge. The rich array of phytochemicals; alkaloids, saponins, and flavonoids and high iron composition seem likely to be responsible for its hematinic effect. Further studies are needed with this plant to evaluate the anti-anaemic active components and to elucidate their mode of action.
DECLARATION
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
Partial support for reagents for phytochemical screening was from the ZAM02 project.
Ethics approval and consent to participate
Ethical clearance was sought from the University of Zambia Biomedical Research Ethics committee (UNZABREC- REF. No: 005-09-16). The experimental protocol was followed according to Guidelines for Care and Use of Laboratory Animals in Biomedical Research (National Research Council (United States) Committee. 2011).
All animals used in this study were anaesthetized with halothane and euthanized by sodium pentobarbital. Remains, including faecal droppings and excess chow were disposed by high temperature incineration at the institutional facility.
ACKNOWLEDGMENTS
The authors would like to thank members of the School of Natural Sciences, Departments of Chemistry, School of Veterinary Medicine, Departments of Disease Control and Biomedical Sciences of the University of Zambia for the equipment and services rendered. The authors also acknowledge support to the Natural Products Research ZAM02 from International Science Programme of Uppsala University, Sweden.
REFERENCES
- Simute S, Phiri CL, Tengnäs B. Agroforestry Extension Manual for Eastern Zambia. Nairobi, Kenya: Majestic Printing Works Ltd.; 1998.
- Burkill HM. The useful plants of West Tropical Africa.Families. E–I. Royal Botanic Gardens, Kew, Richmond, United Kingdom. 1994;2nd Edition. Volume 2:636 pp.
- Schmelzer, Harriët G, Gurib-Fakim, Ameenah. Medicinal plants: Prota; 2008.
- Agyare C, Lechtenberg M, Deters A, Petereit F, Hensel A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: Geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine. 2011;18(7):617-24.
- Boakye YD, Agyare C, Abotsi WKM, Ayande PG, Ossei PPS. Anti-inflammatory activity of aqueous leaf extract of Phyllanthus muellerianus (Kuntze) Exell. and its major constituent, geraniin. Journal of ethnopharmacology. 2016;187:17-27.
- Calixto JB, Santos AR, Filho VC, Yunes RA. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology, and therapeutic potential. Medicinal research reviews. 1998;18(4):225-58.
- Brusotti G, Cesari I, Frassà G, Grisoli P, Dacarro C, Caccialanza G. Antimicrobial properties of stem bark extracts from Phyllanthus muellerianus (Kuntze) Excell. Journal of ethnopharmacology. 2011;135(3):797-800.
- Doughari J, Sunday D. Antibacterial Activity of Phyllanthus muellerianus. Pharmaceutical biology. 2008;46(6):400-5.
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva,Switzerland: World Health Organisation; 2011.
- WHO. Nutritional anaemias: report of a WHO scientific group [meeting held in Geneva from 13 to 17 March 1967]. Geneva, Switzerland.: World Health Organization; 1968.
- WHO. Iron Deficiency Anaemia: Assessment, Prevention, and Control: A Guide for Programme Managers. Geneva, Switzerland.: World Health Organisation; 2001.
- WHO, UNICEF, UNU. Iron deficiency anaemia: assessment, prevention, and control. Geneva, Switzerland: World Health Organisation; 2001.
- Guidelines: Intermittent Iron Supplementation in Preschool and School-age Children. [Internet]. World Health Organisation. 2011.
- De Benoist B, McLean E, Egli I, Cogswell M. WHO global database on anaemia. Geneva: WHO. 2008:1993-2005.
- Murray RK, Granner DK, Mayes PA, Rodwell VW. Happer's Illustrated Biochemistry. USA: Lange medical books / Mc Graw-Hill companies; 2003.
- National Research Council (United States) Committee. Update on the Guide for the Care and Use of Laboratory animals. 2011. In: Guide for the Care and Use of Laboratory animals [Internet]. Washington DC: National Academies Press. 8th. Available from: https://www.ncbi.nlm.nih.gov/books/NBK54052/, .
- Goddard AF, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. 2000;46(suppl 4):iv1-iv5.
- Osungbade KO, Oladunjoye AO. Preventive Treatments of Iron Deficiency Anaemia in Pregnancy: A Review of Their Effectiveness and Implications for Health System Strengthening. Journal of Pregnancy. 2012;2012.
- D.F.I.D. Tackling Maternal and Child Undernutrition in Zambia. A Business Case. Department for International Development - Zambia; 2011.
- N.H.S.P. National Health Strategic Plan 2011-2015. Zambia; 2011.
- Clewes C, Kankasa C, Welsh J, Campbell J. Report of the national survey to evaluate the impact of vitamin A interventions in Zambia in July and November 2003. Atlanta: United States Agency for International Development and CDC; 2003.
- C.S.O. Zambia Demographic and Health Survey 2007. Lusaka; 2009.
- N.F.N.C. Zambia Food Consumption and Micronutrient Status Survey Report 2014.
- USAID. Zambia : Nutrition Profile. 2014.
- Koné W, Koffi A, Bomisso E, Bi FT. Ethnomedical Study and Iron Content of Some Medicinal Herbs Used in Traditional Medicine in Cote D’Ivoire for the Treatment of Anaemia. . African Journal of Traditional, Complementary, and Alternative Medicines. 2012;9(1):81-7.
- Faul F, Erdfelder E, Lang A-G, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39(2):175-91.
- Banda M. Assessing the anti-hyperglycemic and anti-hyperlipidemic effects of an aqueous extract of lannea edulis in alloxan-induced diabetic rats. Lusaka: University of Zambia; 2018.
- Guidelines for the Euthanasia of Animals: 2020 edition [Internet]. 2020. Available from: avma.org.
- Lee H, Blaufox M. Blood volume in the rat. Journal of Nuclear Medicine. 1985;26(1):72-6.
- Banda M, Nyirenda J, Muzandu K, Sijumbila G, Mudenda S. Antihyperglycemic and Antihyperlipidemic Effects of Aqueous Extracts of Lannea edulis in Alloxan-Induced Diabetic Rats. Frontiers in pharmacology. 2018;9:1099.
- Urbaniak GC, & Plous, S. . Research Randomizer (Version 4.0) Computer software 2013.
- Okonkwo CC, U.Njoku O, Mbah MA. Anti-anaemic effect of methanol seed extract of Sphenostylis stenocarpa (African yam bean) in Wistar albino rats. Vol. 7(45), pp. 2907-2913. African Journal of Pharmacy and Pharmacology 2013.
- Oladiji AT, Jacob TO, Yakubu MT. Anti-anaemic potentials of aqueous extract of Sorghum bicolor (L.) moench stem bark in rats. Journal of Ethnopharmacology. 2007;111(3):651-6.
- Stone SH. Method for Obtaining Venous Blood from the Orbital Sinus of the Rat or Mouse. Science (New York, NY). 1954;119(3081):100.
- Bull BS, Simson EV, Assendelft OW. Procedure for determining packed cell volume by the microhaematocrit method. 3rd edition; 20,1-2.1958.
- Thomas D, Hinchliffe R, Briggs C, Macdougall C, Littlewood T, Cavill L. Guideline for the laboratory diagnosis of functional iron deficiency.161: 639-48. Br J Haematol. 2013.
- Trease GE, Evance WC. Pharmacognosy. London: Saunders Publishers; 2002.
- Sofowora A. Medicinal plants and traditional medicine in Africa. Ibadan: Nigeria: Spectrum Books Ltd; 1993. 289-300 p.
- Katsayal UA, Lamai RS. Preliminary phytochemical and antibacterial screening of the ethanolic stem bark extract of Phyllanthus muellerianus. Nig Journal Pharm Sci. 2011;Vol. 8 (No. 2): P. 121-5.
- Yenon AA, Yapi H.F., Gnahoue G., Yapo A.F., Nguessan J. D., Djaman AJ. Anti-anaemic activity of aqueous and ethanolic extracts of Entandrophragma angolense bark on phenylhydrazine- induced anemic rats. Asian Journal of Biochemical and Pharmaceutical Research. 2015;Vol. 5(Issue 3).
- Osman HM, Shayoub M. E., M. BE. The effect of Solenostemma argel on anemia related parameters in Albino Rats and Rabbits. Saudi J Health Sci. 2013;2:81‑6.
- Assob JC, Kamga HL, Nsagha DS, Njunda AL, Nde PF, Asongalem EA, et al. Antimicrobial and toxicological activities of five medicinal plant species from Cameroon Traditional Medicine. BMC complementary and alternative medicine. 2011;11(1):1-11.
- Adedapo AA, Abatan MO, Olorunsogo OO. Effects of some plants of the spurge family on haematological and biochemical parameters in rats. Vet archiv. 2007;77,29-38.
- Okeniyi JO, Loto CA, Popoola AP. Electrochemical performance of Phyllanthus muellerianus on the corrosion of concrete steel-reinforcement in industrial/microbial simulating-environment. Portugaliae Electrochimica Acta. 2014;32(3):199-211.
- Alvarez-Sala A, López-García G, Attanzio A, Tesoriere L, Cilla A, Barberá R, et al. Effects of Plant Sterols or β-Cryptoxanthin at Physiological Serum Concentrations on Suicidal Erythrocyte Death. Journal of Agricultural and Food Chemistry. 2018;66(5):1157-66.
- Shami C, Aman M. Role of flavonoids in the treatment of hemolytic anaemia-a review. European Journal of Pharmaceutical and Medical Research. 2016;3(5):212-6.
Medical Journal of Zambia, Vol 49, 3
The Medical Journal of Zambia, ISSN 0047-651X, is published by the Zambia Medical Association.
© This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.